Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2020-08-11 , DOI: 10.1016/j.yjmcc.2020.07.012 Anthony D Vetter 1 , Ashley A Martin 1 , Brian R Thompson 1 , David D Thomas 2 , Joseph M Metzger 1
|
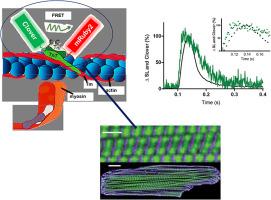
The sarcomere is the functional unit of cardiac muscle, essential for normal heart function. To date, it has not been possible to study, in real time, thin filament-based activation dynamics in live cardiac muscle. We report here results from a cardiac troponin C (TnC) FRET-based biosensor integrated into the cardiac sarcomere via stoichiometric replacement of endogenous TnC. The TnC biosensor provides, for the first time, evidence of multiple thin filament activating ligands, including troponin I interfacing with TnC and cycling myosin, during a cardiac twitch. Results show that the TnC FRET biosensor transient significantly precedes that of peak twitch force. Using small molecules and genetic modifiers known to alter sarcomere activation, independently of the intracellular Ca2+ transient, the data show that the TnC biosensor detects significant effects of the troponin I switch domain as a sarcomere-activating ligand. Interestingly, the TnC biosensor also detected the effects of load-dependent altered myosin cycling, as shown by a significant delay in TnC biosensor transient inactivation during the isometric twitch. In addition, the TnC biosensor detected the effects of myosin as an activating ligand during the twitch by using a small molecule that directly alters cross-bridge cycling, independently of the intracellular Ca2+ transient. Collectively, these results aid in illuminating the basis of cardiac muscle contractile activation with implications for gene, protein, and small molecule-based strategies designed to target the sarcomere in regulating beat-to-beat heart performance in health and disease.
中文翻译:

肌节集成生物传感器在活心肌的抽搐收缩期间实时检测肌丝激活配体。
肌节是心肌的功能单位,对正常心脏功能至关重要。迄今为止,还无法实时研究活心肌中基于细丝的激活动力学。我们在此报告了基于心脏肌钙蛋白 C (TnC) FRET 的生物传感器通过内源性 TnC 的化学计量替代整合到心脏肌节中的结果。TnC 生物传感器首次提供了多种细丝激活配体的证据,包括在心脏抽搐期间与 TnC 和循环肌球蛋白接口的肌钙蛋白 I。结果表明,TnC FRET 生物传感器瞬态显着早于峰值抽搐力。使用已知改变肌节激活的小分子和遗传修饰剂,独立于细胞内 Ca 2+暂时的,数据显示 TnC 生物传感器检测到肌钙蛋白 I 开关域作为肌节激活配体的显着影响。有趣的是,TnC 生物传感器还检测到负载依赖性改变肌球蛋白循环的影响,如等长抽搐期间 TnC 生物传感器瞬态失活的显着延迟所示。此外,TnC 生物传感器通过使用直接改变跨桥循环的小分子,独立于细胞内 Ca 2+检测肌球蛋白在抽搐过程中作为激活配体的作用短暂的。总的来说,这些结果有助于阐明心肌收缩激活的基础,并对旨在针对肌节调节健康和疾病中的逐搏心脏性能的基于基因、蛋白质和小分子的策略产生影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号