Dyes and Pigments ( IF 4.5 ) Pub Date : 2020-08-11 , DOI: 10.1016/j.dyepig.2020.108768 Ami Morimoto , Yuichiro Hayashi , Takeshi Maeda , Shigeyuki Yagi
|
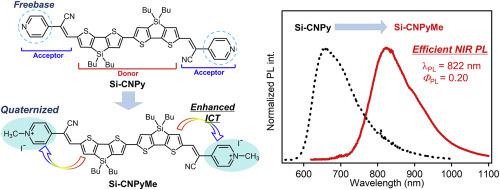
Near-infrared (NIR) emitting functional dyes have been attracting considerable attention because they are useful in the fields of cellular imaging, security application, energy conversion, and so on. In this study, to develop NIR fluorescent dyes with high photoluminescence (PL) quantum yields (ΦPL), novel acceptor–donor–acceptor (A–D–A) type π-conjugated molecules have been prepared, where dimeric dithienosilole (DTS) and freebase or quaternized 1-pyridylacryronitrile are involved as the D and A moieties, respectively. The freebase dye exhibited red fluorescent emission at 654 nm with ΦPL of 0.10 in dichloromethane at rt, whereas the methylation of the pyridine moieties at the molecular termini led to a significant red shift of the emission to the NIR region (PL maximum λPL; 822 nm), accompanied by improvement of a high ΦPL of 0.20 in dichloromethane at rt. The results of density functional theory (DFT) and time-dependent DFT calculations indicated that the considerable red shift of the emission band of the quaternized dye originates from effective intramolecular charge transfer from the DTS donor to the terminal pyridiniums and that the high value of ΦPL is brought about by minimal structural relaxation upon photoexcitation. NIR emission was also obtained when the freebase dye was placed under acidic conditions: protonation at the Lewis basic pyridine moieties brought about halochromic response of PL to yield red-shifted emission (λPL; 755 nm), when trifluoroacetic acid was added to the dichloromethane solution.
中文翻译:

末端路易斯碱性基团调节的A–D–A型功能染料的NIR荧光
由于发射近红外(NIR)的功能性染料在细胞成像,安全性应用,能量转换等领域中很有用,因此受到了极大的关注。在这项研究中,培养NIR具有高的光致发光(PL)量子产率(荧光染料Φ PL),(A-d-A)已经制备型π共轭分子新颖的受体-供体-受体,其中二聚dithienosilole(DTS)和游离碱或季铵化的1-吡啶基氰腈分别作为D和A部分。游离碱染料在654处显示红色荧光发射Φ PL0.10在在室温下的二氯甲烷,而吡啶部分中的导致了发射至NIR区域(PL最大值的显著红移的分子末端的甲基化λ PL ; 822纳米),伴随着改善高的Φ PL的在室温下在二氯甲烷中0.20。密度泛函理论(DFT)和随时间变化的DFT计算结果表明,季铵化染料发射带的相当大的红移源自分子内电荷从DTS供体向末端吡啶鎓的有效转移,并且Φ值较高PL通过光激发最小的结构弛豫来产生“α”。当游离碱染料置于酸性条件下,也获得NIR发射:质子化的路易斯碱的吡啶基部分带来PL的加酸显色反应,以产生红移发射(λ PL ; 755纳米),当加入到二氯甲烷中的三氟乙酸解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号