Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-08-11 , DOI: 10.1016/j.chembiol.2020.07.014 Martin Schröder 1 , Li Tan 2 , Jinhua Wang 3 , Yanke Liang 3 , Nathanael S Gray 3 , Stefan Knapp 4 , Apirat Chaikuad 1
|
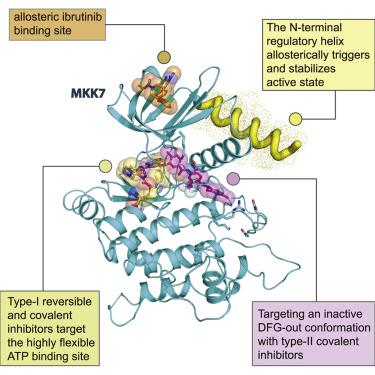
MKK7 (MEK7) is a key regulator of the JNK stress signaling pathway and targeting MKK7 has been proposed as a chemotherapeutic strategy. Detailed understanding of the MKK7 structure and factors that affect its activity is therefore of critical importance. Here, we present a comprehensive set of MKK7 crystal structures revealing insights into catalytic domain plasticity and the role of the N-terminal regulatory helix, conserved in all MAP2Ks, mediating kinase activation. Crystal structures harboring this regulatory helix revealed typical structural features of active kinase, providing exclusively a first model of the MAP2K active state. A small-molecule screening campaign yielded multiple scaffolds, including type II irreversible inhibitors a binding mode that has not been reported previously. We also observed an unprecedented allosteric pocket located in the N-terminal lobe for the approved drug ibrutinib. Collectively, our structural and functional data expand and provide alternative targeting strategies for this important MAP2K kinase.
中文翻译:

MKK7的催化域可塑性揭示了变构活化和多种靶向机会的结构机制。
MKK7(MEK7)是JNK应激信号通路的关键调节剂,已经提出了靶向MKK7作为化疗策略。因此,对MKK7结构和影响其活性的因素的详细了解至关重要。在这里,我们提出了一套完整的MKK7晶体结构,揭示了对催化域可塑性和N末端调节螺旋在所有MAP2K中保守的介导激酶激活的作用的见解。带有这种调节螺旋的晶体结构揭示了活性激酶的典型结构特征,仅提供了MAP2K活性状态的第一个模型。一个小分子的筛选活动产生了多个支架,包括II型不可逆抑制剂,其结合方式此前未见报道。我们还观察到N端叶上有一个空前的变构口袋,用于批准的药物ibrutinib。总的来说,我们的结构和功能数据不断扩展,并为该重要的MAP2K激酶提供了其他靶向策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号