Cell Calcium ( IF 4 ) Pub Date : 2020-08-11 , DOI: 10.1016/j.ceca.2020.102268 Simona Magi 1 , Silvia Piccirillo 1 , Marta Maiolino 1 , Vincenzo Lariccia 1 , Salvatore Amoroso 1
|
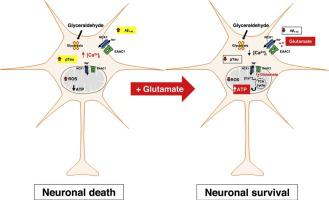
Increasing evidence suggests that metabolic dysfunctions are at the roots of neurodegenerative disorders such as Alzheimer’s disease (AD). In particular, defects in cerebral glucose metabolism, which have been often noted even before the occurrence of clinical symptoms and histopathological lesions, are now regarded as critical contributors to the pathogenesis of AD. Hence, the stimulation of energy metabolism, by enhancing the availability of specific metabolites, might be an alternative way to improve ATP synthesis and to positively affect AD progression. For instance, glutamate may serve as an intermediary metabolite for ATP synthesis through the tricarboxylic acid (TCA) cycle and the oxidative phosphorylation. We have recently shown that two transporters are critical for the anaplerotic use of glutamate: the Na+-dependent Excitatory Amino Acids Carrier 1 (EAAC1) and the Na+-Ca2+ exchanger 1 (NCX1). Therefore, in the present study, we established an AD-like phenotype by perturbing glucose metabolism in both primary rat cortical neurons and retinoic acid (RA)-differentiated SH-SY5Y cells, and we explored the potential of glutamate to halt cell damage by monitoring neurotoxicity, AD markers, ATP synthesis, cytosolic Ca2+ levels and EAAC1/NCX1 functional activities.
We found that glutamate significantly increased ATP production and cell survival, reduced the increase of AD biomarkers (amyloid β protein and the hyperphosphorylated form of tau protein), and recovered the increase of NCX reverse-mode activity. The RNA silencing of either EAAC1 or NCX1 caused the loss of the beneficial effects of glutamate, suggesting the requirement of a functional interplay between these transporters for glutamate-induced protection.
Remarkably, our results indicate, as proof‐of‐principle, that facilitating the use of alternative fuels, like glutamate, may be an effective approach to overcome deficits in glucose utilization and significantly slow down neuronal degenerative process in AD.
中文翻译:

NCX1 和 EAAC1 转运蛋白在体外阿尔茨海默病样模型中参与谷氨酸的保护作用。
越来越多的证据表明,代谢功能障碍是阿尔茨海默病 (AD) 等神经退行性疾病的根源。特别是脑糖代谢缺陷,甚至在临床症状和组织病理学病变出现之前就经常注意到,现在被认为是 AD 发病机制的关键因素。因此,通过提高特定代谢物的可用性来刺激能量代谢,可能是改善 ATP 合成和积极影响 AD 进展的另一种方法。例如,谷氨酸可以作为通过三羧酸 (TCA) 循环和氧化磷酸化进行 ATP 合成的中间代谢物。我们最近表明,两种转运蛋白对于谷氨酸的回补使用至关重要:Na +依赖性兴奋性氨基酸载体 1 (EAAC1) 和 Na + -Ca 2+交换器 1 (NCX1)。因此,在本研究中,我们通过扰乱原代大鼠皮层神经元和视黄酸 (RA) 分化的 SH-SY5Y 细胞中的葡萄糖代谢来建立 AD 样表型,我们通过监测探索了谷氨酸阻止细胞损伤的潜力神经毒性、AD 标志物、ATP 合成、细胞溶质 Ca 2+水平和 EAAC1/NCX1 功能活性。
我们发现谷氨酸显着增加了 ATP 产生和细胞存活,减少了 AD 生物标志物(淀粉样蛋白 β 蛋白和 tau 蛋白的过度磷酸化形式)的增加,并恢复了 NCX 反向模式活性的增加。EAAC1 或 NCX1 的 RNA 沉默导致谷氨酸有益作用的丧失,表明这些转运蛋白之间需要功能性相互作用以实现谷氨酸诱导的保护。
值得注意的是,我们的结果表明,作为原理证明,促进谷氨酸等替代燃料的使用可能是克服葡萄糖利用不足并显着减缓 AD 神经元退行性过程的有效方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号