当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid synthesis of hexahydropyrrolo[3,4-b]pyrrole-fused quinolines via a consecutive [3 + 2] cycloaddition and reduction/intramolecular lactamization cascade
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-10 , DOI: 10.1039/d0qo00858c Yan-Liang Lin 1, 2, 3, 4, 5 , Yun-Ta Lee 1, 2, 3, 4, 5 , Indrajeet J. Barve 1, 2, 3, 4, 5 , Yi-Ting Huang 1, 2, 3, 4, 5 , Chung-Ming Sun 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-10 , DOI: 10.1039/d0qo00858c Yan-Liang Lin 1, 2, 3, 4, 5 , Yun-Ta Lee 1, 2, 3, 4, 5 , Indrajeet J. Barve 1, 2, 3, 4, 5 , Yi-Ting Huang 1, 2, 3, 4, 5 , Chung-Ming Sun 1, 2, 3, 4, 5
Affiliation
|
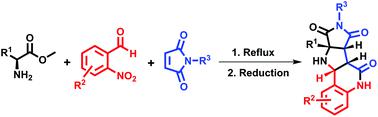
An unprecedented synthesis of novel hexahydropyrrolo[3,4-b]pyrrole-fused quinolines is achieved through the sequential [3 + 2] cycloaddition reaction of azomethine ylides with maleimides followed by intramolecular lactamization. Condensation of the α-amino acid methyl ester with 2-nitrobenzaldehyde leads to an ester stabilized azomethine ylide which further reacts with maleimide to form hexahydropyrrolo[3,4-c]pyrrole. After the reduction of a nitro group, an unusual transamidation reaction is observed, furnishing the novel hexahydropyrrolo[3,4-b]pyrrole-fused quinolines. Isolation of the hexahydropyrrolo[3,4-c]pyrrole amide intermediate revealed the pathway of the reaction mechanism.
中文翻译:

通过连续的[3 + 2]环加成反应和还原/分子内酰胺化级联反应快速合成六氢吡咯并[3,4-b]吡咯稠合的喹啉
通过偶氮甲亚胺与马来酰亚胺的依次[3 + 2]环加成反应,然后进行分子内酰胺化,实现了新型六氢吡咯并[3,4- b ]吡咯稠合的喹啉的空前合成。α-氨基酸甲基酯与2-硝基苯甲醛的缩合产生了酯稳定的偶氮甲碱叶立德,其进一步与马来酰亚胺反应形成六氢吡咯并[3,4- c ]吡咯。硝基还原后,观察到不寻常的氨基转移反应,提供了新颖的六氢吡咯并[3,4- b ]吡咯稠合的喹啉。六氢吡咯并[3,4- c ]吡咯酰胺中间体的分离揭示了反应机理的途径。
更新日期:2020-09-30
中文翻译:

通过连续的[3 + 2]环加成反应和还原/分子内酰胺化级联反应快速合成六氢吡咯并[3,4-b]吡咯稠合的喹啉
通过偶氮甲亚胺与马来酰亚胺的依次[3 + 2]环加成反应,然后进行分子内酰胺化,实现了新型六氢吡咯并[3,4- b ]吡咯稠合的喹啉的空前合成。α-氨基酸甲基酯与2-硝基苯甲醛的缩合产生了酯稳定的偶氮甲碱叶立德,其进一步与马来酰亚胺反应形成六氢吡咯并[3,4- c ]吡咯。硝基还原后,观察到不寻常的氨基转移反应,提供了新颖的六氢吡咯并[3,4- b ]吡咯稠合的喹啉。六氢吡咯并[3,4- c ]吡咯酰胺中间体的分离揭示了反应机理的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号