Medicinal Chemistry ( IF 1.9 ) Pub Date : 2020-07-31 , DOI: 10.2174/1573406415666190614095821 Saira Afzal 1 , Sumera Zaib 1 , Behzad Jafari 2 , Peter Langer 2 , Joanna Lecka 3 , Jean Sévigny 3 , Jamshed Iqbal 1
|
Background: The ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) terminate nucleotide signaling via the hydrolysis of extracellular nucleoside-5'-triphosphate and nucleoside- 5'-diphosphate, to nucleoside-5'-monophosphate and composed of eight Ca2+/Mg2+ dependent ectonucleotidases (NTPDase1-8). Extracellular nucleotides are involved in a variety of physiological mechanisms. However, they are rapidly inactivated by ectonucleotidases that are involved in the sequential removal of phosphate group from nucleotides with the release of inorganic phosphate and their respective nucleoside. Ectonucleoside triphosphate diphosphohydrolases (NTPDases) represent the key enzymes responsible for nucleotides hydrolysis and their overexpression has been related to certain pathological conditions. Therefore, the inhibitors of NTPDases are of particular importance in order to investigate their potential to treat various diseases e.g., cancer, ischemia and other disorders of the cardiovascular and immune system.
Methods: Keeping in view the importance of NTPDase inhibitors, a series of thiadiazolopyrimidones were evaluated for their potential inhibitory activity towards NTPDases by the malachite green assay.
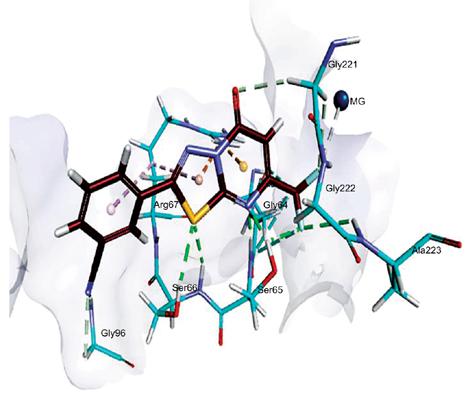
Results: The results suggested that some of the compounds were found as non-selective inhibitors of isozyme of NTPDases, however, most of the compounds act as potent and selective inhibitors. In case of substituted amino derivatives (4c-m), the compounds 4m (IC50 = 1.13 ± 0.09 μM) and 4g (IC50 = 1.72 ± 0.08 μM) were found to be the most potent inhibitors of h-NTPDase1 and 2, respectively. Whereas, compound 4d showed the best inhibitory potential for both h-NTPDase3 (IC50 = 1.25 ± 0.06 μM) and h-NTPDase8 (0.21 ± 0.02 μM). Among 5a-t derivatives, compounds 5e (IC50 = 2.52 ± 0.15 μM), 5p (IC50 = 3.17 ± 0.05 μM), 5n (IC50 = 1.22 ± 0.06 μM) and 5b (IC50 = 0.35 ± 0.001 μM) were found to be the most potent inhibitors of h-NTPDase1, 2, 3 and 8, respectively. Interestingly, the inhibitory concentration values of above-mentioned inhibitors were several folds greater than suramin, a reference control. In order to determine the binding interactions, molecular docking studies of the most potent inhibitors were conducted into the homology models of NTPDases and the putative binding analysis further confirmed that selective and potent compounds bind deep inside the active pocket of the respective enzymes.
Conclusion: The docking analysis proposed that the inhibitory activity correlates with the hydrogen bonds inside the binding pocket. Thus, these derivatives are of interest and may further be investigated for their importance in medicinal chemistry.
中文翻译:

具有2个取代的7-三氟甲基-噻二唑并嘧啶酮骨架的高活性和选择性的外来核苷酸三磷酸二磷酸水解酶(ENTPDase1、2、3和8)抑制剂
背景:胞外核苷三磷酸二磷酸水解酶(NTPDases)通过将胞外5'-三磷酸核苷和5'-二磷酸核苷水解成5'-单磷酸核苷来终止核苷酸信号转导,并由8个依赖Ca2 + / Mg2 +的胞外核苷酸组成( NTPDase1-8)。细胞外核苷酸参与多种生理机制。然而,它们被胞外核苷酸酶快速灭活,该酶涉及从核苷酸中依次除去磷酸基团并释放出无机磷酸盐和它们各自的核苷。核苷三磷酸二磷酸二氢水解酶(NTPDases)代表负责核苷酸水解的关键酶,它们的过表达与某些病理状况有关。因此,
方法:考虑到NTPDase抑制剂的重要性,通过孔雀石绿试验评估了一系列噻二唑并嘧啶酮对NTPDase的潜在抑制活性。
结果:结果表明,发现某些化合物是NTPDases同工酶的非选择性抑制剂,然而,大多数化合物可作为有效和选择性抑制剂。对于取代的氨基衍生物(4c-m),发现化合物4m(IC50 = 1.13±0.09μM)和4g(IC50 = 1.72±0.08μM)分别是h-NTPDase1和2的最强抑制剂。而化合物4d对h-NTPDase3(IC50 = 1.25±0.06μM)和h-NTPDase8(0.21±0.02μM)均显示出最佳的抑制潜力。在5a-t衍生物中,发现化合物5e(IC50 = 2.52±0.15μM),5p(IC50 = 3.17±0.05μM),5n(IC50 = 1.22±0.06μM)和5b(IC50 = 0.35±0.001μM)为h-NTPDase1、2、3和8的最有效抑制剂。有趣的是 上述抑制剂的抑制浓度值是参考对照苏拉明的几倍。为了确定结合相互作用,将最有效的抑制剂的分子对接研究进行了NTPDases的同源性模型的研究,推定的结合分析进一步证实了选择性和有效的化合物深深地结合在相应酶的活性口袋内。
结论:对接分析表明抑制活性与结合口袋内的氢键有关。因此,这些衍生物是令人感兴趣的,并且可以进一步研究它们在药物化学中的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号