Medicinal Chemistry ( IF 1.9 ) Pub Date : 2020-07-31 , DOI: 10.2174/1573406415666190613094433 Chengjun Wu 1 , Jinghan Luo 1 , Mengtong Wu 1 , Fanzhen Meng 1 , Zhiqiang Cai 2 , Yu Chen 3 , Tiemin Sun 1
|
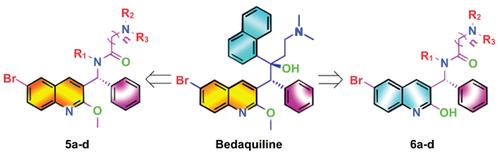
Background: Bedaquiline is a novel anti-tuberculosis drug that inhibits Mycobacterial ATP synthase. However, studies have found that bedaquiline has serious side effects due to high lipophilicity. Recently, the complete structure of ATP synthase was first reported in the Journal of Science.
Objective: The study aimed to design, synthesise and carry out biological evaluation of antituberculosis agents based on the structure of bedaquiline.
Methods: The mode of action of bedaquiline and ATP synthase was determined by molecular docking, and a series of low lipophilic bedaquiline derivatives were synthesized. The inhibitory activities of bedaquiline derivatives towards Mycobacterium phlei 1180 and Mycobacterium tuberculosis H37Rv were evaluated in vitro. A docking study was carried out to elucidate the structureactivity relationship of the obtained compounds. The predicted ADMET properties of the synthesized compounds were also analyzed.
Results: The compounds 5c3, 6a1, and 6d3 showed good inhibitory activities (MIC=15.62 ug.mL-1). At the same time, the compounds 5c3, 6a1, and 6d3 also showed good drug-like properties through molecular docking and ADMET properties prediction.
Conclusion: The results of in vitro anti-tuberculosis activity assays, docking studies and ADMET predictions indicate that the synthesized compounds have potential antifungal activity, with compounds 6a1 being further optimized and developed as lead compounds.
中文翻译:

基于贝达喹啉结构的抗结核药的设计,合成及生物学评价
背景:贝达喹啉是一种新型的抗结核药物,可抑制分枝杆菌ATP合酶。然而,研究发现苯达喹啉由于高度亲脂性而具有严重的副作用。最近,《科学杂志》首次报道了ATP合酶的完整结构。
目的:本研究旨在根据苯达喹啉的结构设计,合成和进行抗结核药物的生物学评估。
方法:通过分子对接确定苯达喹啉和ATP合酶的作用方式,并合成了一系列低亲脂性苯达喹啉衍生物。在体外评价了苯达喹啉衍生物对分枝杆菌1180和结核分枝杆菌H37Rv的抑制活性。进行对接研究以阐明所得化合物的结构活性关系。还分析了合成化合物的预测ADMET性质。
结果:化合物5c3、6a1和6d3表现出良好的抑制活性(MIC = 15.62 ug.mL-1)。同时,化合物5c3、6a1和6d3也通过分子对接和ADMET性质预测显示出良好的类药物性质。
结论:体外抗结核活性测定,对接研究和ADMET预测的结果表明,合成的化合物具有潜在的抗真菌活性,化合物6a1进一步得到了优化并开发为先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号