当前位置:
X-MOL 学术
›
Curr. Genomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Functional characterization of Alr0765, a hypothetical protein from Anabaena PCC 7120 involved in cellular energy status sensing, iron acquisition and abiotic stress management in E. coli using molecular, biochemical and computational approaches
Current Genomics ( IF 2.6 ) Pub Date : 2020-08-08 , DOI: 10.2174/1389202921999200424181239 Antra Chatterjee 1 , Shilpi Singh 1 , Ruchi Rai 1 , Shweta Rai 1 , L.C. Rai 1
Current Genomics ( IF 2.6 ) Pub Date : 2020-08-08 , DOI: 10.2174/1389202921999200424181239 Antra Chatterjee 1 , Shilpi Singh 1 , Ruchi Rai 1 , Shweta Rai 1 , L.C. Rai 1
Affiliation
|
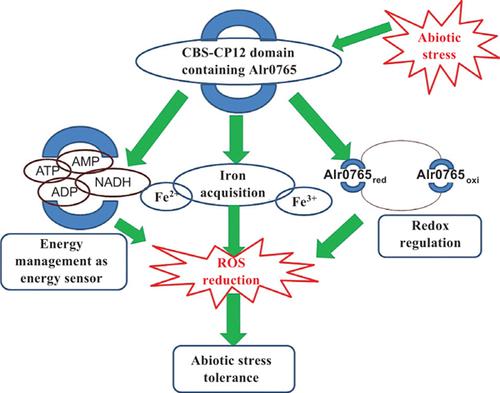
Background: Cyanobacteria are excellent model to understand the basic metabolic processes taking place in response to abiotic stress. The present study involves the characterization of a hypothetical protein Alr0765 of Anabaena PCC7120 comprising the CBS-CP12 domain and deciphering its role in abiotic stress tolerance. Methods: Molecular cloning, heterologous expression and protein purification using affinity chromatography were performed to obtain native purified protein Alr0765. The energy sensing property of Alr0765 was inferred from its binding affinity with different ligand molecules as analyzed by FTIR and TNP-ATP binding assay. AAS and real time-PCR were applied to evaluate the iron acquisition property and cyclic voltammetry was employed to check the redox sensitivity of the target protein. Transcript levels under different abiotic stresses, as well as spot assay, CFU count, ROS level and cellular H2O2 level, were used to show the potential role of Alr0765 in abiotic stress tolerance. In-silico analysis of Alr0765 included molecular function probability analysis, multiple sequence analysis, protein domain and motif finding, secondary structure analysis, protein-ligand interaction, homologous modeling, model refinement and verification and molecular docking was performed with COFACTOR, PROMALS-3D, InterProScan, MEME, TheaDomEx, COACH, Swiss modeller, Modrefiner, PROCHECK, ERRAT, MolProbity, ProSA, TM-align, and Discovery studio, respectively. Results: Transcript levels of alr0765 significantly increased by 20, 13, 15, 14.8, 12, 7, 6 and 2.5 fold when Anabaena PCC7120 treated with LC50 dose of heat, arsenic, cadmium, butachlor, salt, mannitol (drought), UV-B, and methyl viologen respectively, with respect to control (untreated). Heterologous expression resulted in 23KDa protein observed on the SDS-PAGE. Immunoblotting and MALDI-TOF-MS/MS, followed by MASCOT search analysis, confirmed the identity of the protein and ESI/MS revealed that the purified protein was a dimer. Binding possibility of Alr0765 with ATP was observed with an almost 6-fold increment in relative fluorescence during TNP-ATP binding assay with a λ max of 538 nm. FTIR spectra revealed modification in protein confirmation upon binding of Alr0765 with ATP, ADP, AMP and NADH. A 10-fold higher accumulation of iron was observed in digests of E. coli with recombinant vector after induction as compared to control, which affirms the iron acquisition property of the protein. Moreover, the generation of the redox potential of 146 mV by Alr0765 suggested its probable role in maintaining the redox status of the cell under environmental constraints. As per CFU count recombinant, E. coli BL21 cells showed about 14.7, 7.3, 6.9, 1.9, 3 and 4.9 fold higher number of colonies under heat, cadmium (CdCl2), arsenic (Na3AsO4), salt (NaCl), UV-B and drought (mannitol) respectively compared to pET21a harboring E. coli BL21 cells. Deterioration in the cellular ROS level and total cellular H2O2 concentration validated the stress tolerance ability of Alr0765. In-silico analysis unraveled novel findings and attested experimental findings in determining the role of Alr0765. Conclusion: Alr0765 is a novel CBS-CP12 domain protein that maintains cellular energy level and iron homeostasis which provides tolerance against multiple abiotic stresses.
中文翻译:

Alr0765 的功能表征,一种来自鱼腥藻 PCC 7120 的假设蛋白质,使用分子、生化和计算方法参与大肠杆菌的细胞能量状态传感、铁获取和非生物应激管理
背景:蓝藻是了解响应非生物胁迫而发生的基本代谢过程的极好模型。本研究涉及鱼腥藻 PCC7120 的假设蛋白 Alr0765 的表征,该蛋白包含 CBS-CP12 结构域并破译其在非生物胁迫耐受中的作用。方法:通过分子克隆、异源表达和亲和层析纯化蛋白,获得天然纯化蛋白Alr0765。通过 FTIR 和 TNP-ATP 结合测定分析,Alr0765 的能量传感特性是从其与不同配体分子的结合亲和力推断出来的。应用原子吸收光谱法和实时荧光定量 PCR 来评估铁的获取特性,并采用循环伏安法来检查目标蛋白的氧化还原敏感性。不同非生物胁迫下的转录水平,以及斑点测定、CFU 计数、ROS 水平和细胞 H2O2 水平,用于显示 Alr0765 在非生物胁迫耐受中的潜在作用。Alr0765 的计算机分析包括分子功能概率分析、多序列分析、蛋白质结构域和基序发现、二级结构分析、蛋白质-配体相互作用、同源建模、模型细化和验证以及使用 COFACTOR、PROMALS-3D、 InterProScan、MEME、TheaDomEx、COACH、Swiss modeller、Modrefiner、PROCHECK、ERRAT、MolProbity、ProSA、TM-align 和 Discovery studio。结果:鱼腥藻 PCC7120 经 LC50 剂量的热、砷、镉、丁草胺、盐、甘露醇(干旱)、UV-B 和甲基紫精,相对于对照(未处理)。异源表达导致在 SDS-PAGE 上观察到 23KDa 蛋白质。免疫印迹和 MALDI-TOF-MS/MS,然后是 MASCOT 搜索分析,证实了蛋白质的身份,ESI/MS 显示纯化的蛋白质是二聚体。观察到 Alr0765 与 ATP 结合的可能性,在 TNP-ATP 结合测定期间,相对荧光增加了近 6 倍,λ max 为 538 nm。FTIR 光谱揭示了 Alr0765 与 ATP、ADP、AMP 和 NADH 结合后蛋白质确认的改变。与对照相比,在诱导后用重组载体消化的大肠杆菌中观察到铁的积累高 10 倍,这证实了蛋白质的铁获取特性。而且,Alr0765 产生的 146 mV 氧化还原电位表明它可能在环境限制下维持细胞的氧化还原状态中发挥作用。根据重组的 CFU 计数,大肠杆菌 BL21 细胞在加热、镉 (CdCl2)、砷 (Na3AsO4)、盐 (NaCl)、UV-B 下的菌落数量分别增加了约 14.7、7.3、6.9、1.9、3 和 4.9 倍和干旱(甘露醇)分别与含有大肠杆菌 BL21 细胞的 pET21a 进行比较。细胞 ROS 水平和细胞总 H2O2 浓度的恶化验证了 Alr0765 的胁迫耐受能力。计算机分析揭示了新发现并证明了确定 Alr0765 作用的实验结果。结论:Alr0765 是一种新型 CBS-CP12 结构域蛋白,可维持细胞能量水平和铁稳态,提供对多种非生物胁迫的耐受性。
更新日期:2020-08-08
中文翻译:

Alr0765 的功能表征,一种来自鱼腥藻 PCC 7120 的假设蛋白质,使用分子、生化和计算方法参与大肠杆菌的细胞能量状态传感、铁获取和非生物应激管理
背景:蓝藻是了解响应非生物胁迫而发生的基本代谢过程的极好模型。本研究涉及鱼腥藻 PCC7120 的假设蛋白 Alr0765 的表征,该蛋白包含 CBS-CP12 结构域并破译其在非生物胁迫耐受中的作用。方法:通过分子克隆、异源表达和亲和层析纯化蛋白,获得天然纯化蛋白Alr0765。通过 FTIR 和 TNP-ATP 结合测定分析,Alr0765 的能量传感特性是从其与不同配体分子的结合亲和力推断出来的。应用原子吸收光谱法和实时荧光定量 PCR 来评估铁的获取特性,并采用循环伏安法来检查目标蛋白的氧化还原敏感性。不同非生物胁迫下的转录水平,以及斑点测定、CFU 计数、ROS 水平和细胞 H2O2 水平,用于显示 Alr0765 在非生物胁迫耐受中的潜在作用。Alr0765 的计算机分析包括分子功能概率分析、多序列分析、蛋白质结构域和基序发现、二级结构分析、蛋白质-配体相互作用、同源建模、模型细化和验证以及使用 COFACTOR、PROMALS-3D、 InterProScan、MEME、TheaDomEx、COACH、Swiss modeller、Modrefiner、PROCHECK、ERRAT、MolProbity、ProSA、TM-align 和 Discovery studio。结果:鱼腥藻 PCC7120 经 LC50 剂量的热、砷、镉、丁草胺、盐、甘露醇(干旱)、UV-B 和甲基紫精,相对于对照(未处理)。异源表达导致在 SDS-PAGE 上观察到 23KDa 蛋白质。免疫印迹和 MALDI-TOF-MS/MS,然后是 MASCOT 搜索分析,证实了蛋白质的身份,ESI/MS 显示纯化的蛋白质是二聚体。观察到 Alr0765 与 ATP 结合的可能性,在 TNP-ATP 结合测定期间,相对荧光增加了近 6 倍,λ max 为 538 nm。FTIR 光谱揭示了 Alr0765 与 ATP、ADP、AMP 和 NADH 结合后蛋白质确认的改变。与对照相比,在诱导后用重组载体消化的大肠杆菌中观察到铁的积累高 10 倍,这证实了蛋白质的铁获取特性。而且,Alr0765 产生的 146 mV 氧化还原电位表明它可能在环境限制下维持细胞的氧化还原状态中发挥作用。根据重组的 CFU 计数,大肠杆菌 BL21 细胞在加热、镉 (CdCl2)、砷 (Na3AsO4)、盐 (NaCl)、UV-B 下的菌落数量分别增加了约 14.7、7.3、6.9、1.9、3 和 4.9 倍和干旱(甘露醇)分别与含有大肠杆菌 BL21 细胞的 pET21a 进行比较。细胞 ROS 水平和细胞总 H2O2 浓度的恶化验证了 Alr0765 的胁迫耐受能力。计算机分析揭示了新发现并证明了确定 Alr0765 作用的实验结果。结论:Alr0765 是一种新型 CBS-CP12 结构域蛋白,可维持细胞能量水平和铁稳态,提供对多种非生物胁迫的耐受性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号