当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Behavior and Polymorphism of β-Arbutin in Pure and Binary Solvent Systems
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-08-10 , DOI: 10.1021/acs.jced.0c00353 Hui He 1 , Jingxuan Qiu 1 , Haishuang Huang 1 , Haoyou Liu 1, 2 , Shen Hu 1, 2 , Jiaming Han 1, 2 , Dengjing Yi 1, 2 , Mengyao An 2, 3 , Ying Guo 1, 2 , Junxiang Sun 1 , Peng Wang 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-08-10 , DOI: 10.1021/acs.jced.0c00353 Hui He 1 , Jingxuan Qiu 1 , Haishuang Huang 1 , Haoyou Liu 1, 2 , Shen Hu 1, 2 , Jiaming Han 1, 2 , Dengjing Yi 1, 2 , Mengyao An 2, 3 , Ying Guo 1, 2 , Junxiang Sun 1 , Peng Wang 1, 2
Affiliation
|
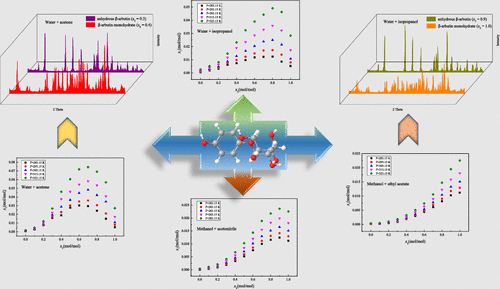
The equilibrium solubility of β-arbutin (ARB) in 12 pure (including water, methanol, ethanol, n-propanol, isopropanol, sec-butanol, n-pentanol, acetone, acetonitrile, ethyl acetate, dichloromethane, and 1,4-dioxane) and 4 binary (water + acetone, water + isopropanol, methanol + acetonitrile, and methanol + ethyl acetate) solvent systems was measured by the gravimetric method at different temperatures under atmospheric pressure. The experimental results indicated that the solubility of ARB increased with the increase of temperature and the content of positive solvent and decreased with the increase of antisolvent composition. The ARB monohydrate was obtained from water and binary solvents containing water, and the cosolvency phenomenon was found in the mixed solvents of water + acetone, water + isopropanol, and methanol + acetonitrile. In addition, the modified Apelblat model, the Jouyban–Acree model, and the Apelblat–Jouyban–Acree model were used to correlate the experimental solubility data, and the values of average relative deviations and root-mean-square deviations between the experimental and calculated solubilities were no more than 11.534 × 10–2 and 18.472 × 10–4, respectively. The calculated values of the three thermodynamic models were in good agreement with the experimental solubility data.
中文翻译:

β-熊果苷在纯和二元溶剂体系中的溶解行为和多态性
β-熊果苷(ARB)在12种纯净水(包括水,甲醇,乙醇,正丙醇,异丙醇,仲丁醇,n-戊醇,丙酮,乙腈,乙酸乙酯,二氯甲烷和1,4-二恶烷)和4种二元体系(水+丙酮,水+异丙醇,甲醇+乙腈和甲醇+乙酸乙酯)通过重量法在90°C下进行测量在大气压下的不同温度。实验结果表明,ARB的溶解度随温度和正溶剂含量的增加而增加,随抗溶剂组成的增加而降低。ARB一水合物是从水和含有水的二元溶剂中获得的,在水+丙酮,水+异丙醇和甲醇+乙腈的混合溶剂中发现了共溶现象。此外,改良的Apelblat模型,Jouyban–Acree模型,–2和18.472×10 –4。三种热力学模型的计算值与实验溶解度数据吻合良好。
更新日期:2020-09-10
中文翻译:

β-熊果苷在纯和二元溶剂体系中的溶解行为和多态性
β-熊果苷(ARB)在12种纯净水(包括水,甲醇,乙醇,正丙醇,异丙醇,仲丁醇,n-戊醇,丙酮,乙腈,乙酸乙酯,二氯甲烷和1,4-二恶烷)和4种二元体系(水+丙酮,水+异丙醇,甲醇+乙腈和甲醇+乙酸乙酯)通过重量法在90°C下进行测量在大气压下的不同温度。实验结果表明,ARB的溶解度随温度和正溶剂含量的增加而增加,随抗溶剂组成的增加而降低。ARB一水合物是从水和含有水的二元溶剂中获得的,在水+丙酮,水+异丙醇和甲醇+乙腈的混合溶剂中发现了共溶现象。此外,改良的Apelblat模型,Jouyban–Acree模型,–2和18.472×10 –4。三种热力学模型的计算值与实验溶解度数据吻合良好。











































 京公网安备 11010802027423号
京公网安备 11010802027423号