当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Site‐Directed Mutagenesis Boosted Selectivity of a Promiscuous Enzyme
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-09 , DOI: 10.1002/adsc.202000604 Pavlína Nekvasilová 1, 2, 3 , Natalia Kulik 4 , Nikola Rychlá 1, 5 , Helena Pelantová 1 , Lucie Petrásková 1 , Zuzana Bosáková 3 , Josef Cvačka 6 , Kristýna Slámová 1 , Vladimír Křen 1 , Pavla Bojarová 1, 5
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-09 , DOI: 10.1002/adsc.202000604 Pavlína Nekvasilová 1, 2, 3 , Natalia Kulik 4 , Nikola Rychlá 1, 5 , Helena Pelantová 1 , Lucie Petrásková 1 , Zuzana Bosáková 3 , Josef Cvačka 6 , Kristýna Slámová 1 , Vladimír Křen 1 , Pavla Bojarová 1, 5
Affiliation
|
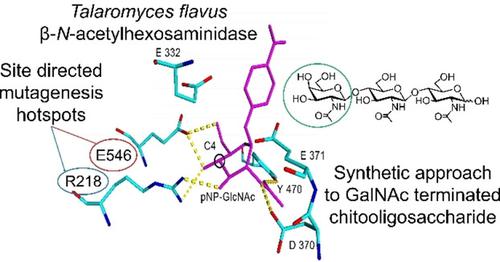
β‐N‐Acetylhexosaminidases (GH20; EC 3.2.1.52) are exo‐glycosidases with a dual activity for cleaving both N‐acetylglucosamine (GlcNAc) and N‐acetylgalactosamine (GalNAc) units from glycostructures. This substrate promiscuity is a hurdle in the selective synthesis of N‐acetylhexosamine oligosaccharides combining both GlcNAc and GalNAc units since there are hardly any GalNAc transferring enzymes available for synthetic applications. We present here site‐directed mutagenesis of a synthetically potent promiscuous β‐N‐acetylhexosaminidase from Talaromyces flavus (TfHex), which, as a wild type, exhibits a GalNAcase/GlcNAcase ratio of 1.2. On the basis of molecular modeling, we identified crucial amino acid residues responsible for its GalNAcase/GlcNAcase selectivity. Six site‐directed mutants were prepared, heterologously expressed in Pichia pastoris, purified, and kinetically characterized. As a result, novel engineered enzymes with an up to 7‐times higher selectivity for either GalNAc or GlcNAc substrates were obtained, preserving the favorable properties of the wild type TfHex, mainly its transglycosylation potential and tolerance to functional groups in the substrate molecule. The substrate selectivity and transglycosylation yield were further corroborated by reaction engineering. The new selective and synthetically capable enzymes were applied in the preparation of tailored N‐acetylhexosamines.
中文翻译:

定点诱变如何提高混杂酶的选择性
β- N-乙酰己糖胺酶(GH20; EC 3.2.1.52)是具有双重活性的外切-糖苷酶,用于从糖结构上裂解N-乙酰氨基葡糖胺(GlcNAc)和N-乙酰半乳糖胺(GalNAc)单元。由于几乎没有任何可用于合成应用的GalNAc转移酶,这种底物的混杂是选择性结合GlcNAc和GalNAc单元的N-乙酰基己糖胺寡糖选择性合成的障碍。我们在这里提出了来自Talaromyces flavus(Tf的合成有效的混杂β- N-乙酰基己糖胺酶的定点诱变(十六进制),其为野生型,其GalNAcase / GlcNAcase比率为1.2。在分子建模的基础上,我们确定了对其GalNAcase / GlcNAcase选择性负责的关键氨基酸残基。制备了六个定点突变体,它们在毕赤酵母中异源表达,纯化并进行了动力学表征。结果,获得了对GalNAc或GlcNAc底物具有高达7倍的高选择性的新型工程化酶,从而保留了野生型TfH的有利特性。例如,主要是其转糖基化潜力和对底物分子中官能团的耐受性。通过反应工程进一步证实了底物选择性和转糖基化产率。新的具有选择性和合成能力的酶被用于制备定制的N-乙酰己糖胺。
更新日期:2020-10-06
中文翻译:

定点诱变如何提高混杂酶的选择性
β- N-乙酰己糖胺酶(GH20; EC 3.2.1.52)是具有双重活性的外切-糖苷酶,用于从糖结构上裂解N-乙酰氨基葡糖胺(GlcNAc)和N-乙酰半乳糖胺(GalNAc)单元。由于几乎没有任何可用于合成应用的GalNAc转移酶,这种底物的混杂是选择性结合GlcNAc和GalNAc单元的N-乙酰基己糖胺寡糖选择性合成的障碍。我们在这里提出了来自Talaromyces flavus(Tf的合成有效的混杂β- N-乙酰基己糖胺酶的定点诱变(十六进制),其为野生型,其GalNAcase / GlcNAcase比率为1.2。在分子建模的基础上,我们确定了对其GalNAcase / GlcNAcase选择性负责的关键氨基酸残基。制备了六个定点突变体,它们在毕赤酵母中异源表达,纯化并进行了动力学表征。结果,获得了对GalNAc或GlcNAc底物具有高达7倍的高选择性的新型工程化酶,从而保留了野生型TfH的有利特性。例如,主要是其转糖基化潜力和对底物分子中官能团的耐受性。通过反应工程进一步证实了底物选择性和转糖基化产率。新的具有选择性和合成能力的酶被用于制备定制的N-乙酰己糖胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号