当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Testicular Lmcd1 regulates phagocytosis by Sertoli cells through modulation of NFAT1/Txlna signaling pathway.
Aging Cell ( IF 8.0 ) Pub Date : 2020-08-09 , DOI: 10.1111/acel.13217 Xiaohang Jin 1 , Sheng Zhang 1 , Tianbing Ding 2 , Pengtao Zhao 1 , Chunli Zhang 1 , Yuxing Zhang 2 , Wei Li 3
Aging Cell ( IF 8.0 ) Pub Date : 2020-08-09 , DOI: 10.1111/acel.13217 Xiaohang Jin 1 , Sheng Zhang 1 , Tianbing Ding 2 , Pengtao Zhao 1 , Chunli Zhang 1 , Yuxing Zhang 2 , Wei Li 3
Affiliation
|
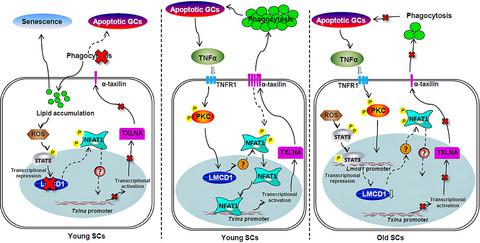
Increased oxidative stress is well known to cause testicular dysfunction in aging males, but the detailed relationships between aging, oxidative stress, and testicular function remain to be elucidated. LIM and cysteine‐rich domains 1 (LMCD1) regulates fundamentally cellular process by interacting with transcription factors. A recent study has identified Lmcd1 as one of the most upregulated nuclear proteins associated with Sertoli cell (SC) differentiation, raising the possibility that testicular actions of LMCD1 are likely to take place. Herein, we reported that LMCD1 was exclusively expressed in the nuclei of SCs. This expression was regulated by TNF‐α signaling produced by apoptotic germ cells (GCs) and was suppressed by oxidative stress in a STAT3‐dependent manner. Ablation of endogenous LMCD1 expression caused lipid accumulation and senescence in GC co‐incubated SCs. Using a previously validated in vivo siRNA approach, we showed that LMCD1 depletion significantly impaired male fertility by inducing oligozoospermia and asthenospermia. Mechanistically, LMCD1 upregulation was associated with the nuclear enrichment of the nuclear factor of activated T cells 1 (NFAT1), a core component of Ca2+/calmodulin‐dependent pathway. LMCD1 facilitated the dephosphorylation and nuclear translocation of NFAT1, which consequently expedited the transactivation of Txlna, a binding partner of the syntaxin family essential for testicular phagocytosis, and thus promoted the removal of apoptotic GCs by phagocytic SCs. Collectively, LMCD1 may operate as a novel pretranscriptional integrator linking SC phagocytosis, lipid homeostasis, and cell senescence.
中文翻译:

睾丸 Lmcd1 通过调节 NFAT1/Txlna 信号通路调节支持细胞的吞噬作用。
众所周知,氧化应激增加会导致老年男性的睾丸功能障碍,但衰老、氧化应激和睾丸功能之间的详细关系仍有待阐明。LIM 和富含半胱氨酸的结构域 1 (LMCD1) 通过与转录因子相互作用从根本上调节细胞过程。最近的一项研究确定了Lmcd1作为与支持细胞 (SC) 分化相关的最上调的核蛋白之一,提高了 LMCD1 可能发生睾丸作用的可能性。在此,我们报道了 LMCD1 仅在 SCs 的细胞核中表达。这种表达受凋亡生殖细胞 (GC) 产生的 TNF-α 信号调节,并以 STAT3 依赖性方式被氧化应激抑制。内源性 LMCD1 表达的消融导致 GC 共孵育的 SCs 中的脂质积累和衰老。使用先前验证的体内siRNA 方法,我们发现 LMCD1 耗竭通过诱导少精子症和弱精子症显着损害男性生育能力。从机制上讲,LMCD1 上调与活化 T 细胞 1 (NFAT1) 核因子的核富集有关,NFAT1 是 Ca 2+ /钙调素依赖途径的核心成分。LMCD1 促进了 NFAT1 的去磷酸化和核易位,从而加速了Txlna的反式激活,Txlna是睾丸吞噬作用所必需的语法蛋白家族的结合伙伴,从而促进了吞噬性 SCs 去除凋亡的 GC。总的来说,LMCD1 可以作为连接 SC 吞噬作用、脂质稳态和细胞衰老的新型转录前整合器。
更新日期:2020-08-09
中文翻译:

睾丸 Lmcd1 通过调节 NFAT1/Txlna 信号通路调节支持细胞的吞噬作用。
众所周知,氧化应激增加会导致老年男性的睾丸功能障碍,但衰老、氧化应激和睾丸功能之间的详细关系仍有待阐明。LIM 和富含半胱氨酸的结构域 1 (LMCD1) 通过与转录因子相互作用从根本上调节细胞过程。最近的一项研究确定了Lmcd1作为与支持细胞 (SC) 分化相关的最上调的核蛋白之一,提高了 LMCD1 可能发生睾丸作用的可能性。在此,我们报道了 LMCD1 仅在 SCs 的细胞核中表达。这种表达受凋亡生殖细胞 (GC) 产生的 TNF-α 信号调节,并以 STAT3 依赖性方式被氧化应激抑制。内源性 LMCD1 表达的消融导致 GC 共孵育的 SCs 中的脂质积累和衰老。使用先前验证的体内siRNA 方法,我们发现 LMCD1 耗竭通过诱导少精子症和弱精子症显着损害男性生育能力。从机制上讲,LMCD1 上调与活化 T 细胞 1 (NFAT1) 核因子的核富集有关,NFAT1 是 Ca 2+ /钙调素依赖途径的核心成分。LMCD1 促进了 NFAT1 的去磷酸化和核易位,从而加速了Txlna的反式激活,Txlna是睾丸吞噬作用所必需的语法蛋白家族的结合伙伴,从而促进了吞噬性 SCs 去除凋亡的 GC。总的来说,LMCD1 可以作为连接 SC 吞噬作用、脂质稳态和细胞衰老的新型转录前整合器。











































 京公网安备 11010802027423号
京公网安备 11010802027423号