当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectrophotometric, Voltammetric and molecular docking studies of binding interaction of N-ferrocenylmethylnitroanilines with bovine serum albumin
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129052 Ali Khennoufa , Lazhar Bechki , Touhami Lanez , Elhafnaoui Lanez , Nadjiba Zegheb
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129052 Ali Khennoufa , Lazhar Bechki , Touhami Lanez , Elhafnaoui Lanez , Nadjiba Zegheb
|
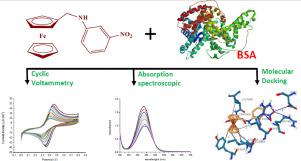
Abstract We have studied the binding interaction of N-ferrocenylmethyl-2-nitroaniline (2FMNA), N-ferrocenylmethyl-3-nitroaniline (3FMNA), and N-ferrocenylmethyl-4-nitroaniline (4FMNA) with bovine serum albumin (BSA) by absorption spectroscopy, cyclic voltammetry, and molecular docking techniques. The results indicated that these derivatives could bind to BSA and cause conformational changes in the order 3FMNA > 2FMNA > 4FMNA. Molecular docking study indicated the preferred binding site, binding mode and further suggested that the binding mode of the three compounds to BSA is of hydrogen bonding and hydrophobic forces, moreover the compound 2FMNA additionally shows a π-π stacking interaction.
中文翻译:

N-二茂铁基甲基硝基苯胺与牛血清白蛋白结合相互作用的分光光度法、伏安法和分子对接研究
摘要 我们研究了 N-二茂铁基甲基-2-硝基苯胺 (2FMNA)、N-二茂铁基甲基-3-硝基苯胺 (3FMNA) 和 N-二茂铁基甲基-4-硝基苯胺 (4FMNA) 与牛血清白蛋白 (BSA) 的结合相互作用。光谱、循环伏安法和分子对接技术。结果表明,这些衍生物可以与 BSA 结合并引起构象变化,顺序为 3FMNA > 2FMNA > 4FMNA。分子对接研究表明了优选的结合位点、结合模式,并进一步表明三种化合物与BSA的结合模式是氢键和疏水力,而且化合物2FMNA还表现出π-π堆积相互作用。
更新日期:2021-01-01
中文翻译:

N-二茂铁基甲基硝基苯胺与牛血清白蛋白结合相互作用的分光光度法、伏安法和分子对接研究
摘要 我们研究了 N-二茂铁基甲基-2-硝基苯胺 (2FMNA)、N-二茂铁基甲基-3-硝基苯胺 (3FMNA) 和 N-二茂铁基甲基-4-硝基苯胺 (4FMNA) 与牛血清白蛋白 (BSA) 的结合相互作用。光谱、循环伏安法和分子对接技术。结果表明,这些衍生物可以与 BSA 结合并引起构象变化,顺序为 3FMNA > 2FMNA > 4FMNA。分子对接研究表明了优选的结合位点、结合模式,并进一步表明三种化合物与BSA的结合模式是氢键和疏水力,而且化合物2FMNA还表现出π-π堆积相互作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号