Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-08-10 , DOI: 10.1016/j.jinorgbio.2020.111209 John J Kozak 1 , Harry B Gray 2 , Roberto A Garza-López 3
|
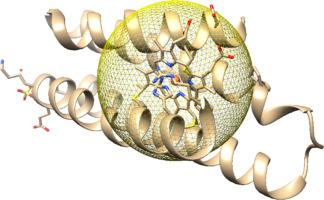
We have analyzed the early stages of unfolding of cytochromes c-b562 (PDB ID: 2BC5) and Rd apo b562 (PDB ID: 1YYJ). Our geometrical approach proceeds from an analysis of the crystal structure reported for each protein. We quantify, residue-by-residue and region-by-region, the spatial and angular changes in the structure as the protein denatures, and quantify differences that result from the seven residues that differ in the two proteins. Using two independent analyses, one based on spatial metrics and the second on angular metrics, we establish the order of unfolding of the five helices in cyt c-b562 and the four helices in the apo protein. For the two helices nearest the N-terminal end of both proteins, the ones in the apo protein unfold first. For the two helices nearest the C-terminal end, the interior helix of the apo protein unfolds first, whereas the terminal helix of the holo protein unfolds first. Excluded-volume effects (repulsive interactions) are minimized in turning regions; the overall range in Δ values is Δ = 36.3 Å3 for cyt c-b562 and Δ = 36.6 Å3 for the apo protein, whereas the span for all 20 amino acids is Δ = 167.7 Å3. As our work indicates that the interior helix of cytochrome c-b562 is the first to fold, we suggest that this helix protects the heme from misligation, consistent with ultrafast folding over a minimally frustrated funneled landscape.
中文翻译:

展开细胞色素 c-b562 和 Rd apo b562。
我们已经分析了细胞色素 cb 562(PDB ID:2BC5)和 Rd apo b 562(PDB ID:1YYJ)展开的早期阶段。我们的几何方法源自对每种蛋白质报告的晶体结构的分析。我们逐个残基和逐个区域量化结构中随着蛋白质变性的空间和角度变化,并量化由两种蛋白质中不同的七个残基引起的差异。使用两个独立的分析,一个基于空间度量,另一个基于角度度量,我们建立了 cyt cb 562 中五个螺旋的展开顺序以及载脂蛋白中的四个螺旋。对于最靠近两种蛋白质 N 末端的两个螺旋,apo 蛋白质中的螺旋首先展开。对于最靠近 C 末端的两个螺旋,apo 蛋白的内部螺旋首先展开,而 holo 蛋白的末端螺旋首先展开。转向区域中的排除体积效应(排斥相互作用)被最小化;Δ 值的总体范围是 Δ = 36.3 Å 3对于 cyt cb 562和 Δ = 36.6 Å 3对于 apo 蛋白,而所有 20 个氨基酸的跨度是 Δ = 167.7 Å 3。由于我们的工作表明细胞色素c -b 562的内部螺旋 是第一个折叠的,我们建议该螺旋保护血红素免于错位,与在最低限度受挫的漏斗状景观上的超快速折叠一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号