European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-08-09 , DOI: 10.1016/j.ejmech.2020.112674 JoLynn B Giancola 1 , Alessandro Bonifazi 1 , Jianjing Cao 1 , Therese Ku 1 , Alexandra J Haraczy 2 , Jenny Lam 2 , Rana Rais 3 , Mark A Coggiano 1 , Gianluigi Tanda 1 , Amy Hauck Newman 1
|
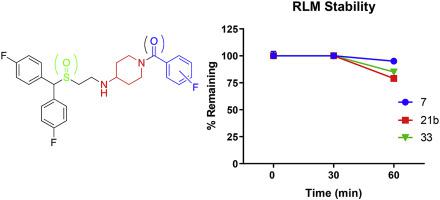
Despite considerable efforts to develop medications to treat psychostimulant use disorders, none have proven effective, leaving an underserved patient population and unanswered questions as to what mechanism(s) of action should be targeted for developing pharmacotherapies. Atypical dopamine transporter (DAT) inhibitors, based on (±)modafinil, have shown therapeutic potential in preclinical models of psychostimulant abuse. However, metabolic instability among other limitations to piperazine analogues 1–3 have impeded further development. Herein, bioisosteric substitutions of the piperazine ring were explored with a series of aminopiperidines (A) and piperidine amines (B) wherein compounds with either a terminal tertiary amine or amide were synthesized. Several lead compounds showed high to moderate DAT affinities and metabolic stability in rat liver microsomes. Aminopiperidines 7 (DAT Ki = 50.6 nM), 21b (DAT Ki = 77.2 nM) and 33 (DAT Ki = 30.0 nM) produced only minimal stimulation of ambulatory activity in mice, compared to cocaine, suggesting an atypical DAT inhibitor profile.
中文翻译:

多巴胺转运蛋白上一系列(双(4-氟苯基)甲基)亚磺酰基乙基-氨基哌啶和-哌啶胺的结构-活性关系:哌嗪的生物等排替代提高了代谢稳定性。
尽管在开发治疗精神兴奋剂使用障碍的药物方面付出了巨大努力,但没有一种药物被证明是有效的,导致患者群体得不到充分服务,并且对于开发药物疗法应针对什么作用机制的问题也没有得到解答。基于(±)莫达非尼的非典型多巴胺转运蛋白(DAT)抑制剂已在精神兴奋剂滥用的临床前模型中显示出治疗潜力。然而,哌嗪类似物1-3的代谢不稳定以及其他限制阻碍了进一步的发展。在此,用一系列氨基哌啶(A)和哌啶胺(B)探索了哌嗪环的生物等排取代,其中合成了具有末端叔胺或酰胺的化合物。几种先导化合物在大鼠肝微粒体中表现出高至中等的 DAT 亲和力和代谢稳定性。与可卡因相比,氨基哌啶7 (DAT K i = 50.6 nM)、 21b (DAT K i = 77.2 nM) 和33 (DAT K i = 30.0 nM) 仅对小鼠的行走活动产生最小的刺激,表明其具有非典型的 DAT 抑制剂特征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号