当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Loops linking secondary structure elements affect the stability of the molten globule intermediate state of apomyoglobin
FEBS Letters ( IF 3.0 ) Pub Date : 2020-09-02 , DOI: 10.1002/1873-3468.13905 Maria A Majorina 1 , Vitaly A Balobanov 1 , Vladimir N Uversky 2, 3 , Bogdan S Melnik 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-09-02 , DOI: 10.1002/1873-3468.13905 Maria A Majorina 1 , Vitaly A Balobanov 1 , Vladimir N Uversky 2, 3 , Bogdan S Melnik 1
Affiliation
|
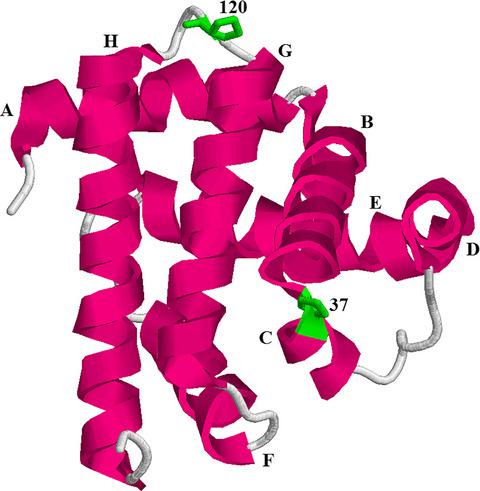
Apomyoglobin is a widely used model for studying the molecular mechanisms of globular protein folding. This work aimed to analyze the effects of rigidity and length of loops linking protein secondary structure elements on the stability of the molten globule intermediate state. For this purpose, we studied folding/unfolding of mutant apomyoglobin forms with substitutions of loop‐located proline residues to glycine and with loop extension by three or six glycine residues. The kinetic and equilibrium experiments performed gave an opportunity to calculate free energies of different apomyoglobin states. Our analysis revealed that the mutations introduced into the apomyoglobin loops have a noticeable effect on the stability of the intermediate state compared to the unfolded state.
中文翻译:

连接二级结构元素的环影响脱脂蛋白肌红蛋白熔球中间态的稳定性
无肌红蛋白是一种广泛使用的模型,用于研究球状蛋白质折叠的分子机制。这项工作旨在分析连接蛋白质二级结构元件的环的刚度和长度对熔球中间态稳定性的影响。为此,我们研究了突变型肌红蛋白形式的折叠/展开,其中环定位的脯氨酸残基替换为甘氨酸,并且环延伸了三个或六个甘氨酸残基。所进行的动力学和平衡实验为计算不同载脂蛋白肌红蛋白状态的自由能提供了机会。我们的分析表明,与未折叠状态相比,引入辅肌红蛋白环的突变对中间状态的稳定性有显着影响。
更新日期:2020-09-02
中文翻译:

连接二级结构元素的环影响脱脂蛋白肌红蛋白熔球中间态的稳定性
无肌红蛋白是一种广泛使用的模型,用于研究球状蛋白质折叠的分子机制。这项工作旨在分析连接蛋白质二级结构元件的环的刚度和长度对熔球中间态稳定性的影响。为此,我们研究了突变型肌红蛋白形式的折叠/展开,其中环定位的脯氨酸残基替换为甘氨酸,并且环延伸了三个或六个甘氨酸残基。所进行的动力学和平衡实验为计算不同载脂蛋白肌红蛋白状态的自由能提供了机会。我们的分析表明,与未折叠状态相比,引入辅肌红蛋白环的突变对中间状态的稳定性有显着影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号