当前位置:
X-MOL 学术
›
Biotechnol. Appl. Bioc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design of a Fe4 S4 cluster into the core of a de novo four-helix bundle.
Biotechnology and Applied Biochemistry ( IF 3.2 ) Pub Date : 2020-08-08 , DOI: 10.1002/bab.2003 Joshua A Mancini 1, 2 , Douglas H Pike 2 , Alexei M Tyryshkin 1 , Liti Haramaty 1 , Michael S Wang 3 , Saroj Poudel 1, 2 , Michael Hecht 3 , Vikas Nanda 2
Biotechnology and Applied Biochemistry ( IF 3.2 ) Pub Date : 2020-08-08 , DOI: 10.1002/bab.2003 Joshua A Mancini 1, 2 , Douglas H Pike 2 , Alexei M Tyryshkin 1 , Liti Haramaty 1 , Michael S Wang 3 , Saroj Poudel 1, 2 , Michael Hecht 3 , Vikas Nanda 2
Affiliation
|
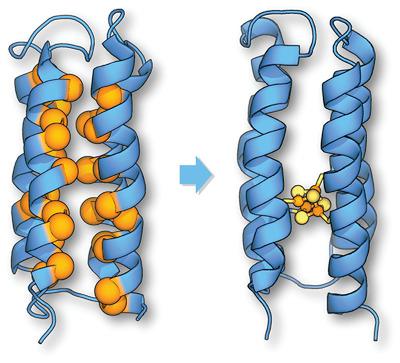
We explore the capacity of the de novo protein, S824, to incorporate a multinuclear iron–sulfur cluster within the core of a single‐chain four‐helix bundle. This topology has a high intrinsic designability because sequences are constrained largely by the pattern of hydrophobic and hydrophilic amino acids, thereby allowing for the extensive substitution of individual side chains. Libraries of novel proteins based on these constraints have surprising functional potential and have been shown to complement the deletion of essential genes in E. coli. Our structure‐based design of four first‐shell cysteine ligands, one per helix, in S824 resulted in successful incorporation of a cubane Fe4S4 cluster into the protein core. A number of challenges were encountered during the design and characterization process, including nonspecific metal‐induced aggregation and the presence of competing metal‐cluster stoichiometries. The introduction of buried iron–sulfur clusters into the helical bundle is an initial step toward converting libraries of designed structures into functional de novo proteins with catalytic or electron‐transfer functionalities.
中文翻译:

将Fe4 S4簇设计为从头开始的四螺旋束的核心。
我们探索了从头蛋白S824在单链四螺旋束核心内整合多核铁硫簇的能力。这种拓扑结构具有很高的固有可设计性,因为序列在很大程度上受到疏水和亲水氨基酸图案的限制,从而允许单个侧链的广泛取代。基于这些限制的新型蛋白质文库具有令人惊讶的功能潜力,并已显示出可补充大肠杆菌中必需基因的缺失。我们在S824中基于结构的四个壳半胱氨酸配体(每个螺旋一个)设计成功地结合了古巴Fe 4 S 4聚集成蛋白质核心。在设计和表征过程中遇到了许多挑战,包括非特异性金属诱导的聚集和竞争性的金属簇化学计量比的存在。将掩埋的铁硫簇引入螺旋束是迈向将设计结构的文库转换为具有催化或电子转移功能的从头蛋白质的第一步。
更新日期:2020-09-10
中文翻译:

将Fe4 S4簇设计为从头开始的四螺旋束的核心。
我们探索了从头蛋白S824在单链四螺旋束核心内整合多核铁硫簇的能力。这种拓扑结构具有很高的固有可设计性,因为序列在很大程度上受到疏水和亲水氨基酸图案的限制,从而允许单个侧链的广泛取代。基于这些限制的新型蛋白质文库具有令人惊讶的功能潜力,并已显示出可补充大肠杆菌中必需基因的缺失。我们在S824中基于结构的四个壳半胱氨酸配体(每个螺旋一个)设计成功地结合了古巴Fe 4 S 4聚集成蛋白质核心。在设计和表征过程中遇到了许多挑战,包括非特异性金属诱导的聚集和竞争性的金属簇化学计量比的存在。将掩埋的铁硫簇引入螺旋束是迈向将设计结构的文库转换为具有催化或电子转移功能的从头蛋白质的第一步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号