当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
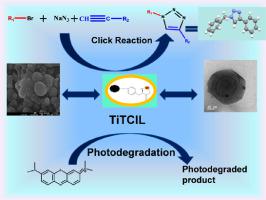
Magnetically separable nanocatalyst (IL@CuFe2O4-L-Tyr-TiO2/TiTCIL): Preparation, Characterization and its applications in 1,2,3-triazole synthesis and in photodegradation of MB
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129029 Neha Sharma , Monika Gupta , Bushra Chowhan , Antonio Frontera
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129029 Neha Sharma , Monika Gupta , Bushra Chowhan , Antonio Frontera
|
Abstract The present work encompasses the synthesis of novel heterogeneous magnetic nanocatalyst(IL@CuFe2O4-L-Tyr-TiO2/TiTCIL) and its characterization by Fourier-transform infrared spectroscopy (FTIR), high-resolution transmission electron microscopy (HR-TEM), field emission gun scanning electron microscopy (FEG-SEM), energy-dispersive X-ray spectroscopy (EDX), vibrating sample magnetometry (VSM), X-ray powder diffraction (P-XRD), X-ray photoelectron spectroscopy (XPS), photoluminescence spectroscopy and Raman spectroscopy. XPS analysis confirms the presence of Cu as Cu1+ and Cu2+ by the effect of the linker in IL@CuFe2O4-L-Tyr-TiO2/TiTCIL. It provides an eco-friendly procedure with several advantages such as operational simplicity, water as the solvent, short reaction time, easy workup and excellent yields in the synthesis of 1,4-disubstituted-1,2,3-triazoles via Click reaction. The catalyst showed recyclability up to seven runs in Click reaction and the recycled catalyst was also characterized by HR-TEM, FEG-SEM and XPS. In Click reaction, one single crystal of 1-benzyl-4-phenyl-1H-1,2,3-triazole was grown. Its energetic features, non-covalent interactions, molecular electrostatic potential surfaces, and packing arrangement were calculated by using the B3LYP-D3/def2-TZVP level of theory and the Bader's quantum theory of "Atoms in molecules" (QTAIM). Moreover, IL@CuFe2O4-L-Tyr-TiO2/TiTCIL also displayed good photocatalytic activity in the degradation of methylene blue dye in visible light.
中文翻译:

磁分离纳米催化剂(IL@CuFe2O4-L-Tyr-TiO2/TiTCIL):制备、表征及其在1,2,3-三唑合成和MB光降解中的应用
摘要 目前的工作包括合成新型多相磁性纳米催化剂 (IL@CuFe2O4-L-Tyr-TiO2/TiTCIL) 及其通过傅里叶变换红外光谱 (FTIR)、高分辨率透射电子显微镜 (HR-TEM)、场发射枪扫描电子显微镜(FEG-SEM)、能量色散X射线光谱(EDX)、振动样品磁强计(VSM)、X射线粉末衍射(P-XRD)、X射线光电子能谱(XPS)、光致发光光谱和拉曼光谱。XPS 分析通过 IL@CuFe2O4-L-Tyr-TiO2/TiTCIL 中接头的作用证实了 Cu 以 Cu1+ 和 Cu2+ 形式存在。它提供了一种环境友好的程序,具有操作简单、以水为溶剂、反应时间短、易于后处理和合成 1,4-二取代-1 的优异收率等优点,通过点击反应生成 2,3-三唑。该催化剂在 Click 反应中显示出最多 7 次运行的可回收性,并且回收的催化剂还通过 HR-TEM、FEG-SEM 和 XPS 进行表征。在 Click 反应中,生长了 1-benzyl-4-phenyl-1H-1,2,3-triazole 的单晶。其能量特征、非共价相互作用、分子静电势面和堆积排列是通过使用 B3LYP-D3/def2-TZVP 理论水平和巴德的“分子中的原子”量子理论(QTAIM)计算的。此外,IL@CuFe2O4-L-Tyr-TiO2/TiTCIL在可见光降解亚甲基蓝染料中也表现出良好的光催化活性。在 Click 反应中,生长了 1-benzyl-4-phenyl-1H-1,2,3-triazole 的单晶。其能量特征、非共价相互作用、分子静电势面和堆积排列是通过使用 B3LYP-D3/def2-TZVP 理论水平和巴德的“分子中的原子”量子理论(QTAIM)计算的。此外,IL@CuFe2O4-L-Tyr-TiO2/TiTCIL在可见光降解亚甲基蓝染料中也表现出良好的光催化活性。在 Click 反应中,生长了 1-benzyl-4-phenyl-1H-1,2,3-triazole 的单晶。其能量特征、非共价相互作用、分子静电势面和堆积排列是通过使用 B3LYP-D3/def2-TZVP 理论水平和巴德的“分子中的原子”量子理论(QTAIM)计算的。此外,IL@CuFe2O4-L-Tyr-TiO2/TiTCIL在可见光降解亚甲基蓝染料中也表现出良好的光催化活性。
更新日期:2021-01-01
中文翻译:

磁分离纳米催化剂(IL@CuFe2O4-L-Tyr-TiO2/TiTCIL):制备、表征及其在1,2,3-三唑合成和MB光降解中的应用
摘要 目前的工作包括合成新型多相磁性纳米催化剂 (IL@CuFe2O4-L-Tyr-TiO2/TiTCIL) 及其通过傅里叶变换红外光谱 (FTIR)、高分辨率透射电子显微镜 (HR-TEM)、场发射枪扫描电子显微镜(FEG-SEM)、能量色散X射线光谱(EDX)、振动样品磁强计(VSM)、X射线粉末衍射(P-XRD)、X射线光电子能谱(XPS)、光致发光光谱和拉曼光谱。XPS 分析通过 IL@CuFe2O4-L-Tyr-TiO2/TiTCIL 中接头的作用证实了 Cu 以 Cu1+ 和 Cu2+ 形式存在。它提供了一种环境友好的程序,具有操作简单、以水为溶剂、反应时间短、易于后处理和合成 1,4-二取代-1 的优异收率等优点,通过点击反应生成 2,3-三唑。该催化剂在 Click 反应中显示出最多 7 次运行的可回收性,并且回收的催化剂还通过 HR-TEM、FEG-SEM 和 XPS 进行表征。在 Click 反应中,生长了 1-benzyl-4-phenyl-1H-1,2,3-triazole 的单晶。其能量特征、非共价相互作用、分子静电势面和堆积排列是通过使用 B3LYP-D3/def2-TZVP 理论水平和巴德的“分子中的原子”量子理论(QTAIM)计算的。此外,IL@CuFe2O4-L-Tyr-TiO2/TiTCIL在可见光降解亚甲基蓝染料中也表现出良好的光催化活性。在 Click 反应中,生长了 1-benzyl-4-phenyl-1H-1,2,3-triazole 的单晶。其能量特征、非共价相互作用、分子静电势面和堆积排列是通过使用 B3LYP-D3/def2-TZVP 理论水平和巴德的“分子中的原子”量子理论(QTAIM)计算的。此外,IL@CuFe2O4-L-Tyr-TiO2/TiTCIL在可见光降解亚甲基蓝染料中也表现出良好的光催化活性。在 Click 反应中,生长了 1-benzyl-4-phenyl-1H-1,2,3-triazole 的单晶。其能量特征、非共价相互作用、分子静电势面和堆积排列是通过使用 B3LYP-D3/def2-TZVP 理论水平和巴德的“分子中的原子”量子理论(QTAIM)计算的。此外,IL@CuFe2O4-L-Tyr-TiO2/TiTCIL在可见光降解亚甲基蓝染料中也表现出良好的光催化活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号