Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-08-08 , DOI: 10.1016/j.bmc.2020.115699 Huan Chen 1 , Xin Zhang 1 , Xiaonan Zhang 1 , Zhenya Fan 1 , Wenchao Liu 1 , Yanqi Lei 1 , Changjin Zhu 1 , Bing Ma 1
|
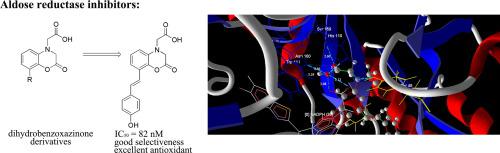
Dihydrobenzoxazinone based design and synthesis produced two series of compounds as aldose reductase (ALR2) inhibitor candidates. In particular, phenolic residues were embodied into the compounds for the combination of strengthening the inhibitory acitvity and antioxidant ability to retard the progression of diabetic complications. Most of the derivatives with styryl side chains exhibited excellent activities on selective ALR2 inhibition with IC50 values ranging from 0.082 to 0.308 μM, and {8-[2-(4-hydroxy-phenyl)-vinyl]-2-oxo-2,3-dihydro-benzo[1,4]oxazin-4-yl}-acetic acid (3a) was the most potent. More significantly, most of dihydrobenzoxazinone compounds revealed not only good inhibitory effect on ALR2, but also showed powerful antioxidant activity. Notably, phenolic compound 3a was even comparable to the well-known antioxidant Trolox, confirming that the C8 p-hydroxystyryl substitution was key structure of lowering oxidative stress. Therefore, these results provided an achievement of multifunctional ALR2 inhibitors possessing capacities for both ALR2 inhibition and as antioxidants.
中文翻译:

二氢苯并恶嗪酮衍生物作为具有抗氧化活性的醛糖还原酶抑制剂
基于二氢苯并恶嗪酮的设计和合成产生了两个系列的化合物作为醛糖还原酶(ALR2)抑制剂候选物。特别地,酚残基被体现在化合物中,以增强抑制活性和抗糖尿病能力以延缓糖尿病并发症的发展。大部分带有苯乙烯基侧链的衍生物表现出优异的选择性ALR2抑制活性,IC 50值范围为0.082至0.308μM ,以及{8- [2-(4-羟基-苯基)-乙烯基] -2-oxo-2, 3-二氢-苯并[1,4]恶嗪-4-基}-乙酸(3a)最有效。更重要的是,大多数二氢苯并恶嗪酮化合物不仅显示出对ALR2的良好抑制作用,而且还显示出强大的抗氧化活性。值得注意的是,酚类化合物3a甚至可以与众所周知的抗氧化剂Trolox相提并论,证实C8对羟基苯乙烯基取代是降低氧化应激的关键结构。因此,这些结果提供了具有同时具有ALR2抑制作用和抗氧化剂作用的多功能ALR2抑制剂的成果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号