当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Allosteric Modulation of Protein Arginine Methyltransferase 5 (PRMT5).
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-08-07 , DOI: 10.1021/acsmedchemlett.9b00525 Rachel L Palte 1 , Sebastian E Schneider 1 , Michael D Altman 1 , Robert P Hayes 2 , Shuhei Kawamura 1 , Brian M Lacey 1 , My Sam Mansueto 1 , Michael Reutershan 1 , Phieng Siliphaivanh 1 , Christopher Sondey 1 , Haiyan Xu 1 , Zangwei Xu 1 , Yingchun Ye 1 , Michelle R Machacek 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-08-07 , DOI: 10.1021/acsmedchemlett.9b00525 Rachel L Palte 1 , Sebastian E Schneider 1 , Michael D Altman 1 , Robert P Hayes 2 , Shuhei Kawamura 1 , Brian M Lacey 1 , My Sam Mansueto 1 , Michael Reutershan 1 , Phieng Siliphaivanh 1 , Christopher Sondey 1 , Haiyan Xu 1 , Zangwei Xu 1 , Yingchun Ye 1 , Michelle R Machacek 1
Affiliation
|
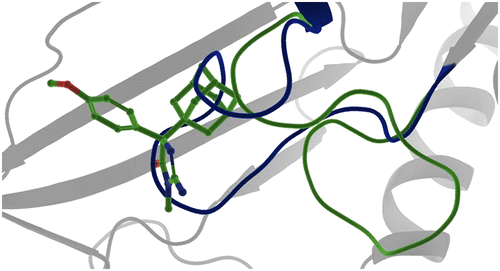
Protein arginine methyltransferase 5 (PRMT5) belongs to a family of enzymes that regulate the posttranslational modification of histones and other proteins via methylation of arginine. Methylation of histones is linked to an increase in transcription and regulates a manifold of functions such as signal transduction and transcriptional regulation. PRMT5 has been shown to be upregulated in the tumor environment of several cancer types, and the inhibition of PRMT5 activity was identified as a potential way to reduce tumor growth. Previously, four different modes of PRMT5 inhibition were known—competing (covalently or non-covalently) with the essential cofactor S-adenosyl methionine (SAM), blocking the substrate binding pocket, or blocking both simultaneously. Herein we describe an unprecedented conformation of PRMT5 in which the formation of an allosteric binding pocket abrogates the enzyme’s canonical binding site and present the discovery of potent small molecule allosteric PRMT5 inhibitors.
中文翻译:

蛋白质精氨酸甲基转移酶 5 (PRMT5) 的变构调节。
蛋白质精氨酸甲基转移酶 5 (PRMT5) 属于一个酶家族,通过精氨酸的甲基化来调节组蛋白和其他蛋白质的翻译后修饰。组蛋白的甲基化与转录增加有关,并调节多种功能,例如信号转导和转录调节。PRMT5 已被证明在几种癌症类型的肿瘤环境中被上调,并且 PRMT5 活性的抑制被确定为减少肿瘤生长的潜在方法。以前,已知四种不同的 PRMT5 抑制模式——与必需辅因子 S-腺苷甲硫氨酸 (SAM) 竞争(共价或非共价)、阻断底物结合口袋或同时阻断两者。
更新日期:2020-09-10
中文翻译:

蛋白质精氨酸甲基转移酶 5 (PRMT5) 的变构调节。
蛋白质精氨酸甲基转移酶 5 (PRMT5) 属于一个酶家族,通过精氨酸的甲基化来调节组蛋白和其他蛋白质的翻译后修饰。组蛋白的甲基化与转录增加有关,并调节多种功能,例如信号转导和转录调节。PRMT5 已被证明在几种癌症类型的肿瘤环境中被上调,并且 PRMT5 活性的抑制被确定为减少肿瘤生长的潜在方法。以前,已知四种不同的 PRMT5 抑制模式——与必需辅因子 S-腺苷甲硫氨酸 (SAM) 竞争(共价或非共价)、阻断底物结合口袋或同时阻断两者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号