当前位置:
X-MOL 学术
›
Batteries Supercaps
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of Magnesium Chloride Complexes in Ethereal Systems: Computational Comparison of THF and Glymes as Solvents for Magnesium Battery Electrolytes
Batteries & Supercaps ( IF 5.1 ) Pub Date : 2020-08-07 , DOI: 10.1002/batt.202000168 Piotr Jankowski 1, 2 , Juan Maria García Lastra 1 , Tejs Vegge 1
Batteries & Supercaps ( IF 5.1 ) Pub Date : 2020-08-07 , DOI: 10.1002/batt.202000168 Piotr Jankowski 1, 2 , Juan Maria García Lastra 1 , Tejs Vegge 1
Affiliation
|
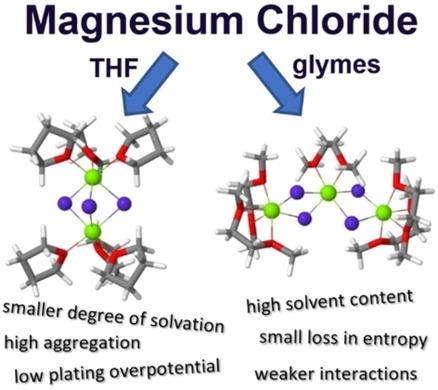
The structure of the electrolyte is crucial for the performance of rechargeable magnesium batteries. Doubly charged cations interact much stronger with both anions and solvent molecules, forming different size clusters. Here, we apply DFT calculations to investigate salt solvation by altering the first solvation shell of the magnesium‐chloride complexes in different ethereal solvents: tetrahydrofuran, monoglyme, diglyme, triglyme and tetraglyme. The analysis was performed by looking for the most stable structures, considering mono‐, di‐ and trimeric clusters of MgxCly. The determination of clusters geometries, together with their energies, resulted in a comprehensive picture of the thermodynamically preferred state of the electrolyte, and allowed for a simple assessment of the electrochemical activity of the electrolyte. Our analysis shows that clustering is beneficial for desolvation of magnesium from the cluster, but causes overpotentials due to hindered electron transfer.
中文翻译:

乙烯系统中氯化镁配合物的结构:作为镁电池电解质溶剂的THF和甘氨酸的计算比较
电解质的结构对于可充电镁电池的性能至关重要。带双电荷的阳离子与阴离子和溶剂分子相互作用更强,形成不同大小的簇。在这里,我们通过改变不同的醚溶剂(四氢呋喃,一甘醇二甲醚,二甘醇二甲醚,三甘醇二甲醚和四甘醇二甲醚)中的氯化镁配合物的第一个溶剂化壳,应用DFT计算来研究盐的溶剂化作用。考虑到Mg x Cl y的单,二和三聚体簇,通过寻找最稳定的结构进行了分析。团簇几何形状及其能量的确定导致了电解质的热力学优选状态的全面描述,并允许简单地评估电解质的电化学活性。我们的分析表明,团簇有利于镁从团簇中脱溶,但由于电子转移受阻而导致过电位。
更新日期:2020-08-07
中文翻译:

乙烯系统中氯化镁配合物的结构:作为镁电池电解质溶剂的THF和甘氨酸的计算比较
电解质的结构对于可充电镁电池的性能至关重要。带双电荷的阳离子与阴离子和溶剂分子相互作用更强,形成不同大小的簇。在这里,我们通过改变不同的醚溶剂(四氢呋喃,一甘醇二甲醚,二甘醇二甲醚,三甘醇二甲醚和四甘醇二甲醚)中的氯化镁配合物的第一个溶剂化壳,应用DFT计算来研究盐的溶剂化作用。考虑到Mg x Cl y的单,二和三聚体簇,通过寻找最稳定的结构进行了分析。团簇几何形状及其能量的确定导致了电解质的热力学优选状态的全面描述,并允许简单地评估电解质的电化学活性。我们的分析表明,团簇有利于镁从团簇中脱溶,但由于电子转移受阻而导致过电位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号