当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coculture techniques for modeling retinal development and disease, and enabling regenerative medicine.
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-08-07 , DOI: 10.1002/sctm.20-0201 Ali E Ghareeb 1, 2 , Majlinda Lako 2 , David H Steel 1, 2
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-08-07 , DOI: 10.1002/sctm.20-0201 Ali E Ghareeb 1, 2 , Majlinda Lako 2 , David H Steel 1, 2
Affiliation
|
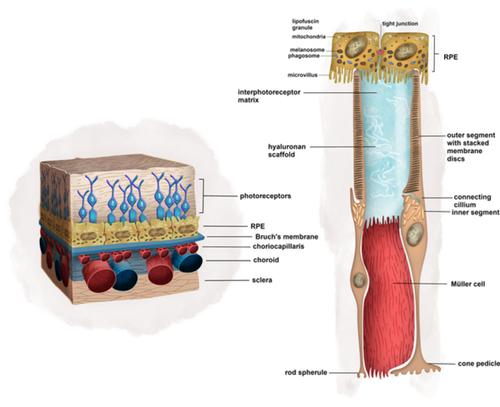
Stem cell‐derived retinal organoids offer the opportunity to cure retinal degeneration of wide‐ranging etiology either through the study of in vitro models or the generation of tissue for transplantation. However, despite much work in animals and several human pilot studies, satisfactory therapies have not been developed. Two major challenges for retinal regenerative medicine are (a) physical cell‐cell interactions, which are critical to graft function, are not formed and (b) the host environment does not provide suitable queues for development. Several strategies offer to improve the delivery, integration, maturation, and functionality of cell transplantation. These include minimally invasive delivery, biocompatible material vehicles, retinal cell sheets, and optogenetics. Optimizing several variables in animal models is practically difficult, limited by anatomical and disease pathology which is often different to humans, and faces regulatory and ethical challenges. High‐throughput methods are needed to experimentally optimize these variables. Retinal organoids will be important to the success of these models. In their current state, they do not incorporate a representative retinal pigment epithelium (RPE)‐photoreceptor interface nor vascular elements, which influence the neural retina phenotype directly and are known to be dysfunctional in common retinal diseases such as age‐related macular degeneration. Advanced coculture techniques, which emulate the RPE‐photoreceptor and RPE‐Bruch's‐choriocapillaris interactions, can incorporate disease‐specific, human retinal organoids and overcome these drawbacks. Herein, we review retinal coculture models of the neural retina, RPE, and choriocapillaris. We delineate the scientific need for such systems in the study of retinal organogenesis, disease modeling, and the optimization of regenerative cell therapies for retinal degeneration.
中文翻译:

用于模拟视网膜发育和疾病并实现再生医学的共培养技术。
干细胞衍生的视网膜类器官通过研究体外模型或生成用于移植的组织,为治疗广泛病因的视网膜变性提供了机会。然而,尽管在动物身上进行了大量工作并进行了多项人体试点研究,但尚未开发出令人满意的治疗方法。视网膜再生医学面临的两个主要挑战是(a)对移植功能至关重要的物理细胞间相互作用尚未形成,以及(b)宿主环境没有提供合适的发育队列。有几种策略可以改善细胞移植的递送、整合、成熟和功能。这些包括微创递送、生物相容性材料载体、视网膜细胞片和光遗传学。优化动物模型中的多个变量实际上很困难,受到解剖学和疾病病理学的限制,而解剖学和疾病病理学通常与人类不同,并且面临监管和伦理挑战。需要高通量方法来通过实验优化这些变量。视网膜类器官对于这些模型的成功非常重要。在目前的状态下,它们不包含代表性的视网膜色素上皮(RPE)-光感受器界面,也不包含血管元件,这些元件直接影响神经视网膜表型,并且已知在常见的视网膜疾病(例如年龄相关性黄斑变性)中存在功能障碍。先进的共培养技术模拟 RPE-光感受器和 RPE-Bruch's-脉络膜毛细血管相互作用,可以合并疾病特异性的人类视网膜类器官并克服这些缺点。在此,我们回顾了神经视网膜、RPE 和脉络膜毛细血管的视网膜共培养模型。 我们描述了在视网膜器官发生、疾病建模和视网膜变性再生细胞疗法优化研究中对此类系统的科学需求。
更新日期:2020-08-07
中文翻译:

用于模拟视网膜发育和疾病并实现再生医学的共培养技术。
干细胞衍生的视网膜类器官通过研究体外模型或生成用于移植的组织,为治疗广泛病因的视网膜变性提供了机会。然而,尽管在动物身上进行了大量工作并进行了多项人体试点研究,但尚未开发出令人满意的治疗方法。视网膜再生医学面临的两个主要挑战是(a)对移植功能至关重要的物理细胞间相互作用尚未形成,以及(b)宿主环境没有提供合适的发育队列。有几种策略可以改善细胞移植的递送、整合、成熟和功能。这些包括微创递送、生物相容性材料载体、视网膜细胞片和光遗传学。优化动物模型中的多个变量实际上很困难,受到解剖学和疾病病理学的限制,而解剖学和疾病病理学通常与人类不同,并且面临监管和伦理挑战。需要高通量方法来通过实验优化这些变量。视网膜类器官对于这些模型的成功非常重要。在目前的状态下,它们不包含代表性的视网膜色素上皮(RPE)-光感受器界面,也不包含血管元件,这些元件直接影响神经视网膜表型,并且已知在常见的视网膜疾病(例如年龄相关性黄斑变性)中存在功能障碍。先进的共培养技术模拟 RPE-光感受器和 RPE-Bruch's-脉络膜毛细血管相互作用,可以合并疾病特异性的人类视网膜类器官并克服这些缺点。在此,我们回顾了神经视网膜、RPE 和脉络膜毛细血管的视网膜共培养模型。 我们描述了在视网膜器官发生、疾病建模和视网膜变性再生细胞疗法优化研究中对此类系统的科学需求。











































 京公网安备 11010802027423号
京公网安备 11010802027423号