当前位置:
X-MOL 学术
›
Surf. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of sugarcane leaf-biomass and investigation of its efficiency in removing Nickel(II), Chromium(III) and Cobalt(II) ions from polluted water
Surfaces and Interfaces ( IF 5.7 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.surfin.2020.100621 Oludoyin Adeseun Adigun , Vincent Olukayode Oninla , N.A. Adesola Babarinde , K.O. Oyedotun , Ncholu Manyala
Surfaces and Interfaces ( IF 5.7 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.surfin.2020.100621 Oludoyin Adeseun Adigun , Vincent Olukayode Oninla , N.A. Adesola Babarinde , K.O. Oyedotun , Ncholu Manyala
|
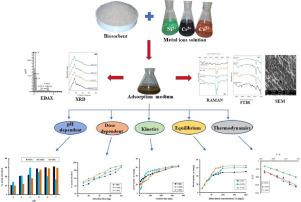
Abstract Sugarcane leaves biomass (SCL) has been utilized for the removal of Ni2+, Cr3+ and Co2+ ions from polluted water. Qualitative analysis was performed by Fourier Transformed Infra-Red spectroscopy, Raman spectroscopy, scanning electron microscopy coupled with energy dispersive spectroscopy and X-ray diffractometry. Adsorption of the metal-ions was carried out by contacting 50 mL of known concentration of Ni2+, Cr3+ and Co2+ with known amount of SCL at 27 °C, and under varied experimental conditions such as pH, ionic strength, solid-to-liquid ratio, contact time and initial metal ions concentration in batch adsorption experiment. The kinetics, isothermal, as well as the nature of the adsorption were predicted by models such as pseudo-first-order, pseudo-second order, Elovich, Weber-Morris, Freundlich, Langmuir, Temkin and Dubinin-Raduschevich. The thermodynamics of the processes was also predicted. Characterization analyses portrayed the surface of SCL as being porous, oval in shape and composed of hydroxyl and carbonyl groups as the main binding sites. Raman analysis revealed the interaction of the metal ions with the lignin, cellulose as well as hemicellulose components of the adsorbent. Adsorption of Ni2+ and Co2+ ions was favored by increasing pH, while that of Cr3+ ions was mostly favored at pH 4. Metal uptake increased with increasing contact time and concentration up to equilibrium stage. The sorption processes followed the pseudo-second-order kinetics and was monolayer in nature with qmax of 51.3, 62.5, and 66.7 mg/g for the uptake of Ni2+, Cr3+ and Co2+ ions, respectively. The models predicted physisorption as the main process involved. Thermodynamic study showed that the processes were feasible and spontaneous, endothermic, and occurred with increase in randomness at the adsorbate-adsorbent interface, demonstrating a good metal uptake nature of the adsorbent.
中文翻译:

甘蔗叶生物量的表征及其从污水中去除镍 (II)、铬 (III) 和钴 (II) 离子的效率研究
摘要 甘蔗叶生物量(SCL)已被用于去除污水中的 Ni2+、Cr3+ 和 Co2+ 离子。通过傅立叶变换红外光谱、拉曼光谱、扫描电子显微镜结合能量色散光谱和X射线衍射进行定性分析。金属离子的吸附是通过将 50 mL 已知浓度的 Ni2+、Cr3+ 和 Co2+ 与已知量的 SCL 在 27 °C 下接触,并在不同的实验条件下进行的,例如 pH、离子强度、固液比、接触时间和间歇吸附实验中的初始金属离子浓度。动力学、等温以及吸附的性质通过诸如伪一级、伪二级、Elovich、Weber-Morris、Freundlich、Langmuir、Temkin 和 Dubinin-Raduschevich。还预测了这些过程的热力学。表征分析将 SCL 的表面描绘成多孔的、椭圆形的并且由羟基和羰基作为主要结合位点组成。拉曼分析揭示了金属离子与吸附剂的木质素、纤维素以及半纤维素组分的相互作用。提高 pH 有利于 Ni2+ 和 Co2+ 离子的吸附,而在 pH 4 时最有利于 Cr3+ 离子的吸附。金属吸收随着接触时间和浓度的增加而增加,直至达到平衡阶段。吸附过程遵循伪二级动力学,本质上是单层的,Ni2+、Cr3+ 和 Co2+ 离子的吸收 qmax 分别为 51.3、62.5 和 66.7 mg/g。这些模型预测物理吸附是所涉及的主要过程。
更新日期:2020-09-01
中文翻译:

甘蔗叶生物量的表征及其从污水中去除镍 (II)、铬 (III) 和钴 (II) 离子的效率研究
摘要 甘蔗叶生物量(SCL)已被用于去除污水中的 Ni2+、Cr3+ 和 Co2+ 离子。通过傅立叶变换红外光谱、拉曼光谱、扫描电子显微镜结合能量色散光谱和X射线衍射进行定性分析。金属离子的吸附是通过将 50 mL 已知浓度的 Ni2+、Cr3+ 和 Co2+ 与已知量的 SCL 在 27 °C 下接触,并在不同的实验条件下进行的,例如 pH、离子强度、固液比、接触时间和间歇吸附实验中的初始金属离子浓度。动力学、等温以及吸附的性质通过诸如伪一级、伪二级、Elovich、Weber-Morris、Freundlich、Langmuir、Temkin 和 Dubinin-Raduschevich。还预测了这些过程的热力学。表征分析将 SCL 的表面描绘成多孔的、椭圆形的并且由羟基和羰基作为主要结合位点组成。拉曼分析揭示了金属离子与吸附剂的木质素、纤维素以及半纤维素组分的相互作用。提高 pH 有利于 Ni2+ 和 Co2+ 离子的吸附,而在 pH 4 时最有利于 Cr3+ 离子的吸附。金属吸收随着接触时间和浓度的增加而增加,直至达到平衡阶段。吸附过程遵循伪二级动力学,本质上是单层的,Ni2+、Cr3+ 和 Co2+ 离子的吸收 qmax 分别为 51.3、62.5 和 66.7 mg/g。这些模型预测物理吸附是所涉及的主要过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号