Environmental Technology & Innovation ( IF 6.7 ) Pub Date : 2020-08-07 , DOI: 10.1016/j.eti.2020.101088 Jiahong Wang , Saleem Atif , Dan Zhang
|
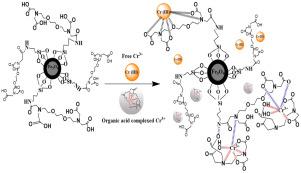
EGTA functionalized magnetic microsphere (Fe3O4@SiO2@EGTA) was prepared for the removal of Cr(III) from highly saline organic wastewater environment. The synthesized adsorbent was characterized with Fourier transform infrared (FTIR), powder x-ray diffraction (XRD), thermogravimetric analysis (TGA) and x-ray photoelectron spectrometry (XPS). The characterization techniques depicted the successful surface modification of magnetic particles (Fe3O4@SiO2) with the functional groups of EGTA without changing its crystal structure. The maximum adsorption amount of Cr(III) was 25.25 mgg−1 at pH 4.0. The Langmuir isotherm was well fitted on the adsorption data, while adsorption equilibrium was achieved in first 15 min, and the equilibrium data well explained by pseudo second order model. The smaller concentrations of inorganic cations (Na, Ca2+, Mg), organic acids (EDTA, Citric acid, Formic acid) and complexing agent with high salt system reduce the adsorption capacity to some extent, but after that the higher concentrations of these competing species have no apparent effect on the adsorption performance. According to the XPS analysis, the carboxyl and amino groups were responsible for the adsorption of Cr(III), while the main mechanism of adsorption were coordination complex and electrostatic interaction. The prepared adsorbent can be readily isolated by external magnetic field and regenerated for several times and still possess higher adsorption performance.
中文翻译:

EGTA修饰的磁性微球对Cr(III)的吸附:高盐度和有机螯合酸的影响
制备了EGTA功能化的磁性微球(Fe 3 O 4 @SiO 2 @EGTA),用于从高盐分有机废水环境中去除Cr(III)。合成的吸附剂通过傅里叶变换红外(FTIR),粉末X射线衍射(XRD),热重分析(TGA)和X射线光电子能谱(XPS)进行表征。表征技术描述了具有EGTA官能团的磁性颗粒(Fe 3 O 4 @SiO 2)的成功表面改性,而没有改变其晶体结构。Cr(III)的最大吸附量为25.25 mg在pH 4.0下为g -1。Langmuir等温线很好地拟合了吸附数据,而在最初的15分钟内就达到了吸附平衡,并且平衡数据可以用伪二阶模型很好地解释。较小浓度的无机阳离子(Na,钙2+,镁),有机酸(EDTA,柠檬酸,甲酸)和高盐体系的络合剂在一定程度上降低了吸附能力,但之后这些竞争物种的较高浓度对吸附性能没有明显影响。根据XPS分析,羧基和氨基是Cr(III)的吸附原因,而吸附的主要机理是配位络合物和静电相互作用。所制备的吸附剂可以很容易地通过外磁场分离并再生数次,仍具有较高的吸附性能。









































 京公网安备 11010802027423号
京公网安备 11010802027423号