Environmental Technology & Innovation ( IF 7.1 ) Pub Date : 2020-08-06 , DOI: 10.1016/j.eti.2020.101085 Vishnu Manirethan , Raj Mohan Balakrishnan
|
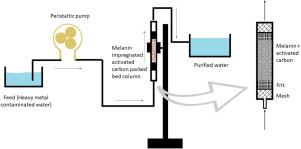
The adversity associated with the removal of melanin nanoparticles after adsorbing heavy metals led to the development of a system involving activated carbon on which melanin was effectively impregnated. The melanin impregnated activated carbon was handily removed from the aqueous medium by simple filtration. The batch studies performed to scrutinise the optimum conditions for maximum adsorption concluded the contact time needed to attain equilibrium to be 90 min for all metals of study. Maximum adsorption of Cr (VI) was shown at pH 3 while Hg (II), Pb (II) and Cu (II) was adsorbed efficiently at pH 5. Increase in temperature favoured the adsorption process for all metals showing maximum removal at 328 K. Melanin impregnated activated carbon could adsorb 84.59% Hg (II), 86.6% Cr (VI), 91.1% Pb (II) and 93.8% Cu (II) from 5 mg/L heavy metal solution. Dynamic studies were conducted by varying the parameters like flow rate, inlet heavy metal concentration and adsorbent loading. Optimum conditions for a maximum breakthrough was obtained with a flow rate of 0.5 mL/min, heavy metal inlet concentration of 1 mg/L and adsorbent loading of 100 mg. Experimental data modelled in equilibrium isotherms showed the best fitting with the Thomas model when compared with the Adam–Boharts model using determined coefficient and error analysis. Efficient chemical desorption of Hg (II), Pb (II) and Cu (II) was obtained using 3N HCl and Cr (VI) using 1N citric acid.
中文翻译:

使用生物合成的黑色素浸渍的活性炭分批和连续研究去除重金属
与吸附重金属后去除黑色素纳米颗粒有关的逆境导致了涉及活性炭的系统的开发,在该系统上有效地浸渍了黑色素。通过简单的过滤,可以轻松地从水性介质中去除浸渍了黑色素的活性炭。为研究最大吸附的最佳条件而进行的分批研究得出结论,对于所有研究金属而言,达到平衡所需的接触时间为90分钟。Cr(VI)在pH 3时最大吸附,而Hg(II),Pb(II)和Cu(II)在pH 5时有效吸附。温度升高有利于所有金属的吸附过程,在328 K时表现出最大去除率黑色素浸渍的活性炭可吸附84.59%的Hg(II),86.6%的Cr(VI),91.1%的Pb(II)和93。来自5 mg / L重金属溶液的8%Cu(II)。通过改变流量,入口重金属浓度和吸附剂负载等参数进行动态研究。以0.5 mL / min的流速,1 mg / L的重金属入口浓度和100 mg的吸附剂负载量获得了最大突破的最佳条件。用确定的系数和误差分析与Adam-Boharts模型进行比较时,以等温等温线为模型的实验数据显示出与Thomas模型的最佳拟合。使用3N HCl和Cr(VI)和1N柠檬酸可实现Hg(II),Pb(II)和Cu(II)的有效化学脱附。5 mL / min,重金属入口浓度为1 mg / L,吸附剂负载为100 mg。用确定的系数和误差分析与Adam-Boharts模型进行比较时,以等温等温线为模型的实验数据显示出与Thomas模型的最佳拟合。使用3N HCl和Cr(VI)和1N柠檬酸可实现Hg(II),Pb(II)和Cu(II)的有效化学脱附。5 mL / min,重金属入口浓度为1 mg / L,吸附剂负载为100 mg。用确定的系数和误差分析与Adam-Boharts模型进行比较时,以等温等温线为模型的实验数据显示出与Thomas模型的最佳拟合。使用3N HCl和Cr(VI)和1N柠檬酸可实现Hg(II),Pb(II)和Cu(II)的有效化学脱附。



























 京公网安备 11010802027423号
京公网安备 11010802027423号