当前位置:
X-MOL 学术
›
Synth. Met.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyaniline coated gold-aryl nanoparticles: Electrochemical synthesis and efficiency in methylene blue dye removal
Synthetic Metals ( IF 4.0 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.synthmet.2020.116528 Buthaina A. AlMashrea , Fatima Abla , Mohamed M. Chehimi , Bizuneh Workie , Changseok Han , Ahmed A. Mohamed
Synthetic Metals ( IF 4.0 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.synthmet.2020.116528 Buthaina A. AlMashrea , Fatima Abla , Mohamed M. Chehimi , Bizuneh Workie , Changseok Han , Ahmed A. Mohamed
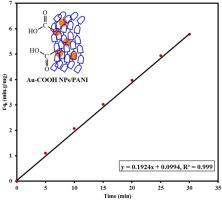
|
Abstract Synthesis of gold-carbon σ-bond nanoparticles was achieved by the electrochemical reduction of aryldiazonium tetrachloroaurate(III) salt [HOOC-4-C6H4N≡N]AuCl4 in the presence of polyaniline (PANI) emeraldine salt. The in situ electrochemical synthesis of PANI coated Au−COOH NPs was efficient in KNO3 supporting electrolyte at different potential values using two electrodes cell. The deposition potential was determined from the cyclic voltammetry study of aryldiazonium gold(III) salt. The nanocomposites were characterized with thermal gravimetric analysis (TGA), transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The multi-branched nanoparticles display the strongest diffraction peak in the direction of (111) which implies the deposition occurred preferentially along this highly energetic facet. The results concluded the thermal stability of the gold nanocomposites and the gold percentage in the range 5−23 wt.%. Selected area electron diffraction pattern (SAED) analysis displayed typical rings of face centered cubic structure of crystalline gold(0) nanoparticles. Raman analysis supported the completeness of the reduction of the aryldiazonium cation and gold(III) supported by the absence of the diazonium and [AuCl4]− vibrations and the presence of the gold-carbon peak. Emeraldine coated gold-carbon nanoparticles soaked to nanosand showed high removal percentage for methylene blue. The adsorption kinetics follows a pseudo-second order. It may indicate that the prepared materials could be used to remove synthetic dyes in textile wastewater treatment.
中文翻译:

聚苯胺包覆的金-芳基纳米粒子:电化学合成和去除亚甲基蓝染料的效率
摘要 通过在聚苯胺(PANI)翡翠盐存在下电化学还原芳基重氮四氯金酸盐[HOOC-4-C6H4N≡N]AuCl4,合成了金碳σ键纳米粒子。使用两个电极电池在不同电位值的 KNO3 支持电解质中,PANI 涂覆的 Au-COOH NPs 的原位电化学合成是有效的。沉积电位由芳基重氮金 (III) 盐的循环伏安法研究确定。通过热重分析 (TGA)、透射电子显微镜 (TEM) 和 X 射线光电子能谱 (XPS) 对纳米复合材料进行表征。多支化纳米粒子在 (111) 方向上显示出最强的衍射峰,这意味着沉积优先沿着这个高能面发生。结果得出结论,金纳米复合材料的热稳定性和金百分比在 5-23 wt.% 的范围内。选区电子衍射图 (SAED) 分析显示结晶金 (0) 纳米粒子的面心立方结构的典型环。拉曼分析支持芳基重氮阳离子和金 (III) 的完全还原,由重氮和 [AuCl4]- 振动的缺失以及金-碳峰的存在支持。浸泡在纳米砂中的祖母绿涂层金碳纳米粒子对亚甲蓝显示出高去除率。吸附动力学遵循伪二级。这可能表明所制备的材料可用于去除纺织废水处理中的合成染料。
更新日期:2020-11-01
中文翻译:

聚苯胺包覆的金-芳基纳米粒子:电化学合成和去除亚甲基蓝染料的效率
摘要 通过在聚苯胺(PANI)翡翠盐存在下电化学还原芳基重氮四氯金酸盐[HOOC-4-C6H4N≡N]AuCl4,合成了金碳σ键纳米粒子。使用两个电极电池在不同电位值的 KNO3 支持电解质中,PANI 涂覆的 Au-COOH NPs 的原位电化学合成是有效的。沉积电位由芳基重氮金 (III) 盐的循环伏安法研究确定。通过热重分析 (TGA)、透射电子显微镜 (TEM) 和 X 射线光电子能谱 (XPS) 对纳米复合材料进行表征。多支化纳米粒子在 (111) 方向上显示出最强的衍射峰,这意味着沉积优先沿着这个高能面发生。结果得出结论,金纳米复合材料的热稳定性和金百分比在 5-23 wt.% 的范围内。选区电子衍射图 (SAED) 分析显示结晶金 (0) 纳米粒子的面心立方结构的典型环。拉曼分析支持芳基重氮阳离子和金 (III) 的完全还原,由重氮和 [AuCl4]- 振动的缺失以及金-碳峰的存在支持。浸泡在纳米砂中的祖母绿涂层金碳纳米粒子对亚甲蓝显示出高去除率。吸附动力学遵循伪二级。这可能表明所制备的材料可用于去除纺织废水处理中的合成染料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号