当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, spectral characteristics, weak interactions, electronic properties and biological activity of (E)-1-(4-hydroxybenzylidene)-4-(3-isopropylphenyl)thiosemicarbazone: An experimental and theoretical approach
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129044 Fan Qi , Jirong Song , Jie Huang
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129044 Fan Qi , Jirong Song , Jie Huang
|
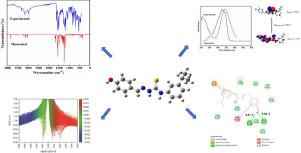
Abstract A novel (E)-1-(4-hydroxybenzylidene)-4-(3-isopropylphenyl)thiosemicarbazone was synthesized and the most stable optimized structure was obtained by using B3LYP/6–31+G(d,p) basis set. The experimental and theoretical researches on the structure, FT-IR spectra, FT-Raman spectra and UV–Visible absorption spectra about the synthetized compound were explored. Reduced density gradient (RDG) function and atoms in molecules (AIM) theory from qualitative and quantitative analysis, respectively, indicated that the intramolecular weak interactions maintained molecular structure stability. The combination of UV-Visible spectra and HOMO-LUMO analysis confirmed the electronic transitions among orbitals. The possible reaction sites of the synthetized compound were predicted by electrostatic potential (ESP) analysis. Molecular docking was used to explore the interactions of the synthetized compound with the antifungal receptor protein (1NMT) at active pocket. Eventually, in vitro antifungal activity of the synthetized compound was evaluated by the disk diffusion method.
中文翻译:

(E)-1-(4-羟基亚苄基)-4-(3-异丙基苯基)缩氨基硫脲的合成、光谱特征、弱相互作用、电子特性和生物活性:实验和理论方法
摘要 合成了一种新型(E)-1-(4-羟基亚苄基)-4-(3-异丙基苯基)缩氨基硫脲,并利用B3LYP/6-31+G(d,p)基组得到了最稳定的优化结构。对合成化合物的结构、傅立叶变换红外光谱、傅立叶变换拉曼光谱和紫外-可见吸收光谱进行了实验和理论研究。分别来自定性和定量分析的降低密度梯度 (RDG) 函数和分子中原子 (AIM) 理论表明,分子内弱相互作用保持了分子结构的稳定性。紫外-可见光谱和 HOMO-LUMO 分析的结合证实了轨道之间的电子跃迁。通过静电势 (ESP) 分析预测合成化合物的可能反应位点。分子对接用于探索合成化合物与活性口袋处的抗真菌受体蛋白 (1NMT) 的相互作用。最后,通过圆盘扩散法评估合成化合物的体外抗真菌活性。
更新日期:2021-01-01
中文翻译:

(E)-1-(4-羟基亚苄基)-4-(3-异丙基苯基)缩氨基硫脲的合成、光谱特征、弱相互作用、电子特性和生物活性:实验和理论方法
摘要 合成了一种新型(E)-1-(4-羟基亚苄基)-4-(3-异丙基苯基)缩氨基硫脲,并利用B3LYP/6-31+G(d,p)基组得到了最稳定的优化结构。对合成化合物的结构、傅立叶变换红外光谱、傅立叶变换拉曼光谱和紫外-可见吸收光谱进行了实验和理论研究。分别来自定性和定量分析的降低密度梯度 (RDG) 函数和分子中原子 (AIM) 理论表明,分子内弱相互作用保持了分子结构的稳定性。紫外-可见光谱和 HOMO-LUMO 分析的结合证实了轨道之间的电子跃迁。通过静电势 (ESP) 分析预测合成化合物的可能反应位点。分子对接用于探索合成化合物与活性口袋处的抗真菌受体蛋白 (1NMT) 的相互作用。最后,通过圆盘扩散法评估合成化合物的体外抗真菌活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号