当前位置:
X-MOL 学术
›
Int. J. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Losses of CO and CO2 upon collision-activated dissociation of substituted 2-methoxyphenoxides after methyl radical loss
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.ijms.2020.116397 Christopher L. Marcum , James S. Riedeman , Tiffany M. Jarrell , John J. Nash , Hilkka I. Kenttämaa
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.ijms.2020.116397 Christopher L. Marcum , James S. Riedeman , Tiffany M. Jarrell , John J. Nash , Hilkka I. Kenttämaa
|
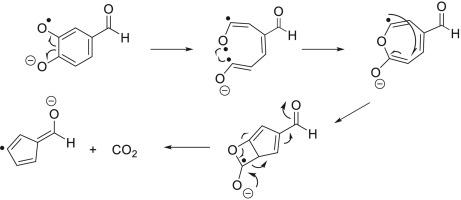
Abstract Some deprotonated, substituted 2-methoxyphenols fragment by methyl radical loss followed by competing losses of CO and CO2 upon collisional activation in linear quadrupole ion trap mass spectrometers. These reactions were examined experimentally and computationally in order to determine their mechanisms. For deprotonated vanillin, the CO loss was found to involve ring contraction, with the highest free energy barrier of 58.0 kcal mol−1 (for the syn rotamer; here, syn refers to the relationship between the aldehyde oxygen atom and the oxygen atom of the methoxy group) for the entire process. The atoms lost in this fragmentation are the oxygen atom that was bound to the first eliminated methyl group and the aromatic carbon atom bound to this oxygen. Examination of carbon-13 labeled vanillin supports these assignments. Examination of several model compounds revealed that this reaction requires the presence of an electron-withdrawing substituent in the para-position relative to the phenol moiety. In contrast, the CO2 loss from deprotonated vanillin occurs via ring opening followed by re-cyclization and then ring contraction, leading to the loss of CO2 in a process wherein the highest free energy barrier is 84.5 kcal mol−1 (for the syn isomer). The atoms lost in this fragmentation are the carbon and oxygen atoms from the phenoxide group and the oxygen atom that was bound to the first eliminated methyl group, which is supported by examination of carbon-13 labeled vanillin. Despite the higher total free energy requirement, the CO2 loss is competitive with CO loss, possibly due to favorable entropy and low activation energy (free energy barrier of 48.9 kcal mol−1) for the first reaction step (the analogous value for CO loss is 58.0 kcal mol−1). The extent of CO2 loss is strongly affected by substituents – it either is not observed or is very slow for the other compounds studied here. For example, it is substantially less favorable than CO loss (highest free energy barrier 60.9 kcal mol−1) for deprotonated acetovanillone for which the first reaction step for CO2 loss has a substantially greater free energy barrier (60.6 kcal mol−1) than for vanillin.
中文翻译:

甲基自由基损失后取代的 2-甲氧基苯氧化物碰撞激活解离后 CO 和 CO2 的损失
摘要 在线性四极杆离子阱质谱仪中,在碰撞活化时,一些去质子化、取代的 2-甲氧基苯酚碎片由于甲基自由基损失,然后是 CO 和 CO2 的竞争损失。这些反应通过实验和计算进行了检查,以确定它们的机制。对于去质子化香草醛,发现 CO 损失涉及环收缩,最高自由能垒为 58.0 kcal mol-1(对于同位异构体;这里,syn 指醛氧原子和氧原子之间的关系)甲氧基)整个过程。在这种断裂中丢失的原子是与第一个消除的甲基结合的氧原子和与该氧结合的芳族碳原子。对碳 13 标记的香草醛的检查支持这些分配。对几种模型化合物的检查表明,该反应需要在相对于苯酚部分的对位存在吸电子取代基。相比之下,去质子化香草醛的 CO2 损失通过开环、再环化和环收缩发生,导致在最高自由能垒为 84.5 kcal mol-1(对于顺式异构体)的过程中损失 CO2 . 在这种碎裂中丢失的原子是来自苯氧基团的碳和氧原子,以及与第一个消除的甲基结合的氧原子,碳 13 标记的香草醛的检查支持了这一点。尽管总自由能要求较高,但 CO2 损失与 CO 损失竞争,这可能是由于有利的熵和低活化能(自由能垒为 48. 9 kcal mol-1) 用于第一个反应步骤(CO 损失的类似值为 58.0 kcal mol-1)。CO2 损失的程度受到取代基的强烈影响——对于这里研究的其他化合物,要么没有观察到,要么非常缓慢。例如,对于去质子化乙酰香兰酮来说,它比 CO 损失(最高自由能垒 60.9 kcal mol-1)要差得多,对于 CO2 损失的第一个反应步骤的自由能垒(60.6 kcal mol-1)比香草醛。
更新日期:2020-10-01
中文翻译:

甲基自由基损失后取代的 2-甲氧基苯氧化物碰撞激活解离后 CO 和 CO2 的损失
摘要 在线性四极杆离子阱质谱仪中,在碰撞活化时,一些去质子化、取代的 2-甲氧基苯酚碎片由于甲基自由基损失,然后是 CO 和 CO2 的竞争损失。这些反应通过实验和计算进行了检查,以确定它们的机制。对于去质子化香草醛,发现 CO 损失涉及环收缩,最高自由能垒为 58.0 kcal mol-1(对于同位异构体;这里,syn 指醛氧原子和氧原子之间的关系)甲氧基)整个过程。在这种断裂中丢失的原子是与第一个消除的甲基结合的氧原子和与该氧结合的芳族碳原子。对碳 13 标记的香草醛的检查支持这些分配。对几种模型化合物的检查表明,该反应需要在相对于苯酚部分的对位存在吸电子取代基。相比之下,去质子化香草醛的 CO2 损失通过开环、再环化和环收缩发生,导致在最高自由能垒为 84.5 kcal mol-1(对于顺式异构体)的过程中损失 CO2 . 在这种碎裂中丢失的原子是来自苯氧基团的碳和氧原子,以及与第一个消除的甲基结合的氧原子,碳 13 标记的香草醛的检查支持了这一点。尽管总自由能要求较高,但 CO2 损失与 CO 损失竞争,这可能是由于有利的熵和低活化能(自由能垒为 48. 9 kcal mol-1) 用于第一个反应步骤(CO 损失的类似值为 58.0 kcal mol-1)。CO2 损失的程度受到取代基的强烈影响——对于这里研究的其他化合物,要么没有观察到,要么非常缓慢。例如,对于去质子化乙酰香兰酮来说,它比 CO 损失(最高自由能垒 60.9 kcal mol-1)要差得多,对于 CO2 损失的第一个反应步骤的自由能垒(60.6 kcal mol-1)比香草醛。











































 京公网安备 11010802027423号
京公网安备 11010802027423号