Insect Biochemistry and Molecular Biology ( IF 3.8 ) Pub Date : 2020-08-08 , DOI: 10.1016/j.ibmb.2020.103454 Yuanyuan Zhou , Wenlan Wang , Nahiyan Mohammad Salauddin , Lianyun Lin , Minsheng You , Shijun You , Zhiguang Yuchi
|
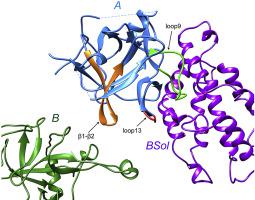
Ryanodine receptors (RyRs) are the molecular target of diamides, a new chemical class of insecticides. Diamide insecticides are used to control lepidopteran pests and were considered relatively safe for mammals and non-targeted beneficial insects, including honey bees. However, recent studies showed that exposure to diamides could cause long-lasting locomotor deficits of bees. Here we report the crystal structure of RyR N-terminal domain A (NTD-A) from the honeybee, Apis mellifera, at 2.5 Å resolution. It shows a similar overall fold as the RyR NTD-A from mammals and the diamondback moth (DBM), Plutella xylostella, and still several loops located at the inter-domain interfaces show insect-specific or bee-specific structural features. A potential insecticide-binding pocket formed by loop9 and loop13 is conserved in lepidopteran but different in both mammals and bees, making it a good candidate targeting site for the development of pest-selective insecticides. Furthermore, a conserved intra-domain disulfide bond was observed in both DBM and bee RyR NTD-A crystal structures, which explains their higher thermal stability compared to mammalian RyR NTD-A. This work provides a basis for the development of novel insecticides with better selectivity between pests and bees by targeting a distinct site on pest RyRs, which would be a promising strategy to overcome the current toxicity problem.
中文翻译:

蜜蜂Apis mellifera ryanodine受体N末端结构域的晶体结构。
Ryanodine受体(RyRs)是二酰胺(一种新型的化学杀虫剂)的分子靶标。二酰胺杀虫剂用于防治鳞翅目害虫,被认为对哺乳动物和非定向有益昆虫(包括蜜蜂)相对安全。但是,最近的研究表明,暴露于二酰胺可能会导致蜜蜂的长期运动缺陷。在这里,我们报告了蜜蜂Apis mellifera的RyR N末端域A(NTD-A)的晶体结构,分辨率为2.5。它显示出与来自哺乳动物和小菜蛾(Plutella xylostella)的RyR NTD-A相似的总体折叠,而且位于域间界面的几个环仍显示出昆虫特定或蜜蜂特定的结构特征。由loop9和loop13形成的潜在的杀虫剂结合袋在鳞翅目中被保留,但在哺乳动物和蜜蜂中都不同,这使其成为开发害虫选择性杀虫剂的良好候选靶点。此外,在DBM和蜜蜂RyR NTD-A晶体结构中均观察到保守的域内二硫键,这说明与哺乳动物RyR NTD-A相比,它们具有更高的热稳定性。这项工作通过针对害虫RyRs上的一个独特位点,为开发在害虫和蜜蜂之间具有更好选择性的新型杀虫剂提供了基础,这将是克服当前毒性问题的一种有前途的策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号