Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-08-08 , DOI: 10.1016/j.bmc.2020.115671 Chengsheng Chen 1 , Cristin Bosko 1 , Catherine P McGeough 1 , Ryan McLean 1 , Angela M Zaino 2 , M Kyle Hadden 2 , Mark W Peczuh 1
|
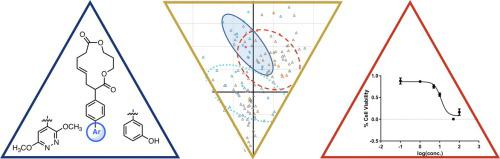
A macrocyclic motif fosters productive protein-small molecule interactions. There are numerous examples of both natural product and designed, synthetic macrocycles that modulate the immune system, slow microbial infection, or kill eukaryotic cells. Reported here are the synthesis, physicochemical characterization, and antiproliferative activity of a group of [13]-macrodilactones decorated with a pendant biaryl moiety. Biaryl analogs were prepared by Suzuki reactions conducted on a common intermediate that contained a bromophenyl unit alpha to one of the carbonyls of the [13]-macrodilactone. Principal component analysis placed the new compounds in physicochemical context relative to a variety of pharmaceuticals and natural products. Modest inhibition of proliferation was observed in ASZ cells, a murine basal cell carcinoma line. This work underscores the value of an approach toward the identification of bioactive compounds that places the evaluation of physicochemical parameters early in the search process.
中文翻译:

探索联芳基连接的[13]-宏二内酯的理化和抗增殖特性
大环基元促进生产性蛋白质-小分子的相互作用。天然产物和设计的合成大环化合物都有许多实例,它们调节免疫系统,减缓微生物感染或杀死真核细胞。此处报道的是一组由侧基联芳基修饰的[13]-宏二内酯的合成,理化性质和抗增殖活性。通过在常见中间体上进行的Suzuki反应制得联芳基类似物,该中间体含有与[13]-大二内酯的羰基之一相对的溴苯基单元。主成分分析将这些新化合物置于理化背景下,相对于多种药物和天然产品而言。在鼠基础细胞癌系ASZ细胞中观察到适度的增殖抑制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号