Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-08-08 , DOI: 10.1016/j.bbamem.2020.183436 Paulo Roberto Dores-Silva 1 , David M Cauvi 2 , Vanessa T R Kiraly 3 , Júlio C Borges 3 , Antonio De Maio 4
|
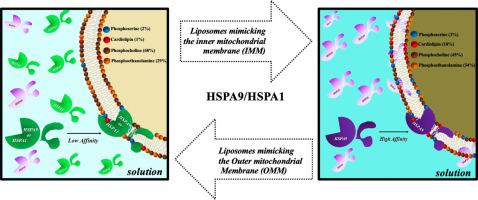
Mitochondrial Hsp70 (HSPA9, mtHsp70, mortalin) in conjunction with a complex set of other proteins is involved in the transport of polypeptides across the mitochondrial matrix. This observation allows us to hypothesize that HSPA9 might interact with membranes directly, similarly to other Hsp70s. Thus, we investigated whether human HSPA9 could also get inserted into lipid membranes. Human HSPA9 was incubated with liposomes made of lipids found within the mitochondrial membrane, such as 1′, 3′-bis [1, 2-dimyristoyl-sn-glycero-3-phospho]-glycerol (CL), palmitoyl-oleoyl phosphocholine (POPC), palmitoyl-oleoyl phosphoserine (POPS), and palmitoyl-oleoyl phosphoethanolamine (POPE). HSPA9 displayed a predilection for CL and POPS, and low affinity for POPC and POPE, suggesting that the proteins have high specificity for negatively charged phospholipids. Then, liposomes were made with a composition resembling either the outer or inner mitochondrial membrane (OMM or IMM, respectively). We observed that HSPA9 has a higher affinity for IMM than OMM, which is consistent with the higher content of CL in the IMM. A comparison for the incorporation into POPS or CL liposomes by HSPA9 or HSPA1 indicated that both proteins behaved very similarly when exposed to CL liposomes, but differently with POPS liposomes, which was further corroborated by their susceptibility to proteinase K digestion after incorporation into liposomes. The measurement of thermodynamic parameters also showed that the interaction of both proteins with CL and POPS liposomes was different. Overall, our data showed that HSPA9 is prone to interact with membranes resembling the IMM that may be important for its role in the translocation of proteins into the mitochondria.
中文翻译:

人HSPA9(mtHsp70,莫塔林)与含有心磷脂的脂质双层相互作用,心磷脂是线粒体内膜的主要成分。
线粒体Hsp70(HSPA9,mtHsp70,mortalin)与一组复杂的其他蛋白质结合在一起,参与了跨线粒体基质的多肽运输。该观察结果使我们可以假设HSPA9可能与其他Hsp70相似,直接与膜相互作用。因此,我们调查了人类HSPA9是否也可以插入脂质膜中。将人HSPA9与由线粒体膜内存在的脂质制成的脂质体温育,例如1',3'-双[1,2-二肉豆蔻酰基-sn-甘油3-磷酸]-甘油(CL),棕榈酰-油酰磷酸胆碱( POPC),棕榈酰基-油酰基磷酸丝氨酸(POPS)和棕榈酰基-油酰基磷酸乙醇胺(POPE)。HSPA9显示出对CL和POPS的偏爱,对POPC和POPE的亲和力低,表明该蛋白对带负电荷的磷脂具有高特异性。然后,用类似于线粒体外膜或内线膜(分别为OMM或IMM)的组合物制备脂质体。我们观察到HSPA9对IMM的亲和力比OMM高,这与IMM中CL的含量较高相符。通过HSPA9或HSPA1将其掺入POPS或CL脂质体的比较表明,两种蛋白质在暴露于CL脂质体时的行为非常相似,但与POPS脂质体却表现出不同,这进一步证实了它们在掺入脂质体后对蛋白酶K消化的敏感性。热力学参数的测量还表明,两种蛋白质与CL和POPS脂质体的相互作用是不同的。总体,











































 京公网安备 11010802027423号
京公网安备 11010802027423号