Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-08-07 , DOI: 10.1016/j.actbio.2020.07.061 Mengmeng Long 1 , Ailing Lu 1 , Min Lu 1 , Lingyan Weng 1 , Qiuping Chen 2 , Li Zhu 1 , Zhongping Chen 1
|
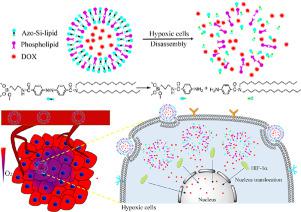
Stimuli-responsive drug delivery systems using endogenous stimuli from tumor microenvironments such as acidic pH, over-expressed enzyme, and high redox potential as triggers have shown tremendous promise in cancer therapy. However, their clinical application is severely limited because of tumor heterogeneity. Hypoxia, a physiological feature observed in almost all solid tumors and even in nodules with very small size, has currently emerged as a more general but efficient stimulus to trigger release. Herein, we developed hypoxia-responsive hybrid liposomes (HR-HLPs), composed of azo-inserted organokoxysilane-based lipid analogue as a responsive component and commercial phospholipid for reducing the rigidity of liposomal membrane caused by azo, for drug delivery targeting tumor hypoxia. HR-HLPs had the advantages of high structural stability to avoid premature drug leakage when circulating in the blood and high sensitivity in responding to hypoxia once reaching tumor sites. HR-HLPs exhibit deep tumor penetration capability, enabling effective delivery to hypoxic regions distant from tumor vessels. Moreover, HR-HLPs could selectively release their payload, co-localizing with over-expressed hypoxia inducible factor 1α (HIF-1α) in vitro and in vivo. As a result, HR-HLPs showed improved therapeutic outcome accompanied by reduced adverse effects. The results highlighted the potential application of azo-inserted responsive hybrid liposomes for hypoxia-targeted drug delivery.
Statement of Significance
- 1.
A hypoxia-responsive hybrid liposomal drug delivery system is developed for specific drug delivery targeting tumor hypoxia.
- 2.
The hybrid liposomes have high structural stability to avoid premature drug leakage under normal physiological condition and high sensitivity in responding to hypoxia once reaching tumor sites.
- 3.
The hybrid liposomes exhibit deep tumor penetration capability, enabling effective delivery to hypoxic regions distant from tumor vessels and co-localization with over-expressed HIF-1α in vitro and in vivo.
中文翻译:

偶氮插入的响应性混合脂质体用于缺氧特异性药物递送。
使用来自肿瘤微环境的内源性刺激(例如酸性pH,过表达的酶和高氧化还原电势)作为诱因的刺激响应药物传递系统在癌症治疗中显示出巨大的希望。然而,由于肿瘤的异质性,其临床应用受到严重限制。缺氧是几乎在所有实体瘤甚至小结节中观察到的一种生理特征,目前已成为触发释放的更普遍但有效的刺激手段。本文中,我们开发了缺氧反应性混合脂质体(HR-HLP),由偶氮插入的基于有机基氧基硅烷的脂质类似物作为反应性成分和商用磷脂组成,可降低由偶氮引起的脂质体膜的刚性,用于靶向肿瘤缺氧的药物递送。HR-HLPs具有较高的结构稳定性,可避免在血液中循环时药物过早泄漏,并且一旦到达肿瘤部位,对缺氧的反应高度敏感。HR-HLPs具有很强的肿瘤穿透能力,能够有效递送到远离肿瘤血管的低氧区域。此外,HR-HLP可以选择性释放其有效负载,并与过表达的缺氧诱导因子1α(HIF-1α)共定位 体外和体内。结果,HR-HLPs显示出改善的治疗效果,同时减少了不良反应。结果强调了偶氮插入的响应性混合脂质体在低氧靶向药物递送中的潜在应用。
重要声明
- 1。
开发了一种低氧反应性混合脂质体药物递送系统,用于靶向肿瘤缺氧的特异性药物递送。
- 2。
杂合脂质体具有很高的结构稳定性,可以避免在正常生理条件下药物过早泄漏,并且一旦到达肿瘤部位就对缺氧有很高的敏感性。
- 3。
杂合脂质体具有深层的肿瘤穿透能力,能够在体外和体内有效递送至远离肿瘤血管的低氧区域,并与过度表达的HIF-1α共定位。









































 京公网安备 11010802027423号
京公网安备 11010802027423号