当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Brønsted-acid-catalyzed one-pot tandem annulation/[5 + 2]-cycloaddition of o-propargyl alcohol benzaldehydes with alkynes: regioselective and stereoselective synthesis of dibenzo[a,f]azulen-12-ones
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-08-06 , DOI: 10.1039/d0qo00522c Ni-Ni Zhou 1, 2, 3, 4, 5 , Si-Si Ning 1, 2, 3, 4, 5 , Lin-Qiang Li 1, 2, 3, 4, 5 , Jie-Yun Zhang 1, 2, 3, 4, 5 , Ming-Jin Fan 1, 2, 3, 4, 5 , De-Suo Yang 1, 2, 3, 4, 5 , Hai-Tao Zhu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-08-06 , DOI: 10.1039/d0qo00522c Ni-Ni Zhou 1, 2, 3, 4, 5 , Si-Si Ning 1, 2, 3, 4, 5 , Lin-Qiang Li 1, 2, 3, 4, 5 , Jie-Yun Zhang 1, 2, 3, 4, 5 , Ming-Jin Fan 1, 2, 3, 4, 5 , De-Suo Yang 1, 2, 3, 4, 5 , Hai-Tao Zhu 1, 2, 3, 4, 5
Affiliation
|
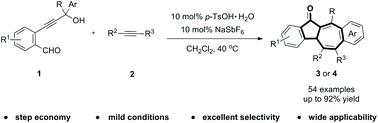
The one-pot synthesis of dibenzo[a,f]azulen-12-ones has been established starting from o-propargyl alcohol benzaldehydes and alkynes. The key azulenone bicyclic skeletons were formed through the intramolecular tandem cyclization and intermolecular [5 + 2]-cycloaddition sequence. This annulation process showed high atom and step economy, regioselectivity and stereoselectivity by creating three C–C and one C–O bonds.
中文翻译:

布朗斯台德酸催化一锅串联环化/邻炔丙醇苯甲醛与炔烃的[5 + 2]-环加成反应:区域选择性和立体选择性合成二苯并[a,f] azulen-12-ones
从邻-炔丙基醇苯甲醛和炔烃开始,已经建立了一锅合成二苯并[ a,f ] azulen-12-ones 。关键的天青酮双环骨架是通过分子内串联环化和分子间[5 + 2]-环加成序列形成的。通过形成三个C–C和一个C–O键,这种成环过程显示出较高的原子和步长经济性,区域选择性和立体选择性。
更新日期:2020-09-16
中文翻译:

布朗斯台德酸催化一锅串联环化/邻炔丙醇苯甲醛与炔烃的[5 + 2]-环加成反应:区域选择性和立体选择性合成二苯并[a,f] azulen-12-ones
从邻-炔丙基醇苯甲醛和炔烃开始,已经建立了一锅合成二苯并[ a,f ] azulen-12-ones 。关键的天青酮双环骨架是通过分子内串联环化和分子间[5 + 2]-环加成序列形成的。通过形成三个C–C和一个C–O键,这种成环过程显示出较高的原子和步长经济性,区域选择性和立体选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号