当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological screening of a library of macamides as TNF-α inhibitors
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-08-06 , DOI: 10.1039/d0md00208a Víctor Tena Pérez 1 , Luis Apaza Ticona 1, 2 , Andreea Madalina Serban 3 , Javier Acero Gómez 1 , Ángel Rumbero Sánchez 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-08-06 , DOI: 10.1039/d0md00208a Víctor Tena Pérez 1 , Luis Apaza Ticona 1, 2 , Andreea Madalina Serban 3 , Javier Acero Gómez 1 , Ángel Rumbero Sánchez 1
Affiliation
|
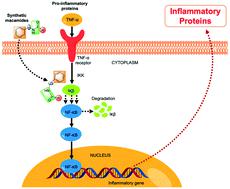
Thirty-five macamide analogues were synthesised by modifying the initial molecular structure. The resulting structures were confirmed using NMR and MS. Cytotoxicity and the anti-inflammatory activity of these synthetic macamides were evaluated in the THP-1 cell line. Preliminary biological evaluation indicated that most of these synthetic macamides did not present cytotoxicity (MTT assay) in the tested cell line with respect to the control (actinomycin D). Regarding the anti-inflammatory activity, several analogues had a greater potential for inhibition of TNF-α than natural macamides. Synthetic macamide 4a was the most active (IC50 = 0.009 ± 0.001 μM) compared to the C87 (control). Through looking at the link between the chemical structure and the activity, our study proves that changes made to natural macamides at the level of the alkyl chain, the benzyl position, the amide bond, and the addition of two methyl groups to the aromatic ring (meta position) lead us to obtaining new macamides with greater anti-inflammatory activity.
中文翻译:

作为 TNF-α 抑制剂的 macamides 文库的合成和生物筛选
通过修改初始分子结构合成了 35 个 macamide 类似物。使用NMR和MS确认所得结构。在 THP-1 细胞系中评估了这些合成 macamides 的细胞毒性和抗炎活性。初步生物学评估表明,与对照(放线菌素 D)相比,这些合成的 macamides 中的大多数在测试细胞系中不存在细胞毒性(MTT 测定)。关于抗炎活性,一些类似物比天然马酰胺具有更大的抑制 TNF-α 的潜力。合成的 macamide 4a是最活跃的 (IC 50= 0.009 ± 0.001 μM) 与 C87(对照)相比。通过观察化学结构和活性之间的联系,我们的研究证明了天然大麦酰胺在烷基链、苄基位置、酰胺键以及在芳环上添加两个甲基的水平发生了变化(元位置)引导我们获得具有更大抗炎活性的新macamides。
更新日期:2020-08-06
中文翻译:

作为 TNF-α 抑制剂的 macamides 文库的合成和生物筛选
通过修改初始分子结构合成了 35 个 macamide 类似物。使用NMR和MS确认所得结构。在 THP-1 细胞系中评估了这些合成 macamides 的细胞毒性和抗炎活性。初步生物学评估表明,与对照(放线菌素 D)相比,这些合成的 macamides 中的大多数在测试细胞系中不存在细胞毒性(MTT 测定)。关于抗炎活性,一些类似物比天然马酰胺具有更大的抑制 TNF-α 的潜力。合成的 macamide 4a是最活跃的 (IC 50= 0.009 ± 0.001 μM) 与 C87(对照)相比。通过观察化学结构和活性之间的联系,我们的研究证明了天然大麦酰胺在烷基链、苄基位置、酰胺键以及在芳环上添加两个甲基的水平发生了变化(元位置)引导我们获得具有更大抗炎活性的新macamides。











































 京公网安备 11010802027423号
京公网安备 11010802027423号