当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequential Suzuki-Miyaura Coupling/Lewis Acid-Catalyzed Cyclization: An Entry to Functionalized Cycloalkane-Fused Naphthalenes.
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-05 , DOI: 10.1021/acs.orglett.0c02020 Camilo Mahecha-Mahecha 1, 2 , Frédéric Lecornué 1 , Sumita Akinari 1, 3 , Thomas Charote 1 , Diego Gamba-Sánchez 2 , Tomohiko Ohwada 3 , Sébastien Thibaudeau 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-05 , DOI: 10.1021/acs.orglett.0c02020 Camilo Mahecha-Mahecha 1, 2 , Frédéric Lecornué 1 , Sumita Akinari 1, 3 , Thomas Charote 1 , Diego Gamba-Sánchez 2 , Tomohiko Ohwada 3 , Sébastien Thibaudeau 1
Affiliation
|
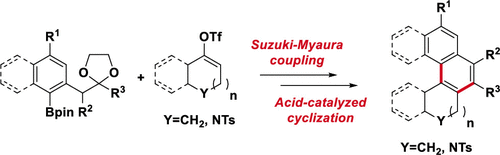
Functionalized angular cycloalkane-fused naphthalenes were prepared using a two-step process involving a Pd-catalyzed Suzuki–Miyaura coupling of aryl pinacol boronates and vinyl triflates followed by a boron trifluoride etherate-catalyzed cycloaromatization.
中文翻译:

顺序铃木-宫浦偶联剂/路易斯酸催化的环化:功能化的环烷烃基萘的入口。
使用两步法制备官能化的有角环烷烃稠合萘,该方法包括钯催化的芳基频哪醇硼酸酯和乙烯基三氟甲磺酸酯的Suzuki-Miyaura偶联,然后是三氟化硼醚化物催化的环芳构化。
更新日期:2020-08-21
中文翻译:

顺序铃木-宫浦偶联剂/路易斯酸催化的环化:功能化的环烷烃基萘的入口。
使用两步法制备官能化的有角环烷烃稠合萘,该方法包括钯催化的芳基频哪醇硼酸酯和乙烯基三氟甲磺酸酯的Suzuki-Miyaura偶联,然后是三氟化硼醚化物催化的环芳构化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号