当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel Biphasic Lipolysis Method To Predict in Vivo Performance of Lipid-Based Formulations.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.molpharmaceut.0c00427 Patrick J O'Dwyer 1, 2, 3 , Karl J Box 1 , Niklas J Koehl 3 , Harriet Bennett-Lenane 3 , Christos Reppas 2 , Rene Holm 4, 5 , Martin Kuentz 6 , Brendan T Griffin 3
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.molpharmaceut.0c00427 Patrick J O'Dwyer 1, 2, 3 , Karl J Box 1 , Niklas J Koehl 3 , Harriet Bennett-Lenane 3 , Christos Reppas 2 , Rene Holm 4, 5 , Martin Kuentz 6 , Brendan T Griffin 3
Affiliation
|
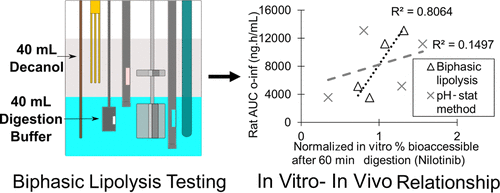
The absence of an intestinal absorption sink is a significant weakness of standard in vitro lipolysis methods, potentially leading to poor prediction of in vivo performance and an overestimation of drug precipitation. In addition, the majority of the described lipolysis methods only attempt to simulate intestinal conditions, thus overlooking any supersaturation or precipitation of ionizable drugs as they transition from the acidic gastric environment to the more neutral conditions of the intestine. The aim of this study was to develop a novel lipolysis method incorporating a two-stage gastric-to-intestinal transition and an absorptive compartment to reliably predict in vivo performance of lipid-based formulations (LBFs). Drug absorption was mimicked by in situ quantification of drug partitioning into a decanol layer. The method was used to characterize LBFs from four studies described in the literature, involving three model drugs (i.e., nilotinib, fenofibrate, and danazol) where in vivo bioavailability data have previously been reported. The results from the novel biphasic lipolysis method were compared to those of the standard pH-stat method in terms of reliability for predicting the in vivo performance. For three of the studies, the novel biphasic lipolysis method more reliably predicted the in vivo bioavailability compared to the standard pH-stat method. In contrast, the standard pH-stat method was found to produce more predictive results for one study involving a series of LBFs composed of the soybean oil, glyceryl monolinoleate (Maisine CC), Kolliphor EL, and ethanol. This result was surprising and could reflect that increasing concentrations of ethanol (as a cosolvent) in the formulations may have resulted in greater partitioning of the drug into the decanol absorptive compartment. In addition to the improved predictivity for most of the investigated systems, this biphasic lipolysis method also uses in situ analysis and avoids time- and resource-intensive sample analysis steps, thereby facilitating a higher throughput capacity and biorelevant approach for characterization of LBFs.
中文翻译:

预测脂类制剂体内性能的新型双相脂解方法。
肠道吸收池的缺乏是标准体外脂解方法的显着弱点,可能导致对体内性能的不良预测以及对药物沉淀的高估。另外,所描述的大多数脂解方法仅试图模拟肠的状况,因此当可电离的药物从酸性胃环境过渡到肠的中性状况时,会忽略任何可离子化的药物的过饱和或沉淀。这项研究的目的是开发一种新的脂解方法,该方法结合了一个两阶段的胃到肠过渡和吸收间隔,以可靠地预测基于脂质的制剂(LBF)的体内性能。药物吸收被模仿原位定量分配到癸醇层中的药物。该方法用于表征文献中描述的四项研究中的LBF,涉及三种模型药物(即尼洛替尼,非诺贝特和达那唑),以前已经报道了体内生物利用度数据。就预测体内性能的可靠性而言,将新型双相脂肪分解方法的结果与标准pH静态方法的结果进行了比较。对于其中的三项研究,新型双相脂解方法可以更可靠地预测体内与标准pH-stat方法相比的生物利用度。相反,对于一项涉及一系列由大豆油,单油酸甘油酯(Maisine CC),Kolliphor EL和乙醇组成的LBF的研究,发现标准的pH-stat方法可产生更多的预测结果。该结果是令人惊讶的,并且可以反映出制剂中乙醇(作为助溶剂)浓度的增加可能导致药物在癸醇吸收室中的分配更大。除了改进了大多数研究系统的可预测性外,这种双相脂解方法还使用了原位分析,并避免了时间和资源密集的样品分析步骤,从而促进了更高的通量能力和生物相关方法来表征LBF。
更新日期:2020-09-09
中文翻译:

预测脂类制剂体内性能的新型双相脂解方法。
肠道吸收池的缺乏是标准体外脂解方法的显着弱点,可能导致对体内性能的不良预测以及对药物沉淀的高估。另外,所描述的大多数脂解方法仅试图模拟肠的状况,因此当可电离的药物从酸性胃环境过渡到肠的中性状况时,会忽略任何可离子化的药物的过饱和或沉淀。这项研究的目的是开发一种新的脂解方法,该方法结合了一个两阶段的胃到肠过渡和吸收间隔,以可靠地预测基于脂质的制剂(LBF)的体内性能。药物吸收被模仿原位定量分配到癸醇层中的药物。该方法用于表征文献中描述的四项研究中的LBF,涉及三种模型药物(即尼洛替尼,非诺贝特和达那唑),以前已经报道了体内生物利用度数据。就预测体内性能的可靠性而言,将新型双相脂肪分解方法的结果与标准pH静态方法的结果进行了比较。对于其中的三项研究,新型双相脂解方法可以更可靠地预测体内与标准pH-stat方法相比的生物利用度。相反,对于一项涉及一系列由大豆油,单油酸甘油酯(Maisine CC),Kolliphor EL和乙醇组成的LBF的研究,发现标准的pH-stat方法可产生更多的预测结果。该结果是令人惊讶的,并且可以反映出制剂中乙醇(作为助溶剂)浓度的增加可能导致药物在癸醇吸收室中的分配更大。除了改进了大多数研究系统的可预测性外,这种双相脂解方法还使用了原位分析,并避免了时间和资源密集的样品分析步骤,从而促进了更高的通量能力和生物相关方法来表征LBF。











































 京公网安备 11010802027423号
京公网安备 11010802027423号