当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Isotope Effects in Electrophilic Aromatic Halogenation of Dimethenamid in Chlor(am)inated Water Demonstrate Unique Aspects of Iodination
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.estlett.0c00557 Michael R. Rose 1, 2 , Keith P. Reber 3
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2020-08-06 , DOI: 10.1021/acs.estlett.0c00557 Michael R. Rose 1, 2 , Keith P. Reber 3
Affiliation
|
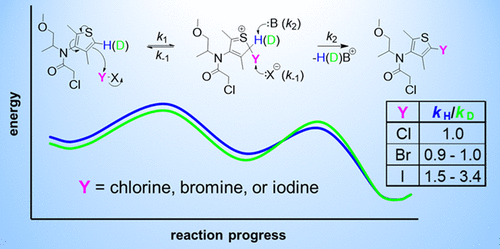
Electrophilic aromatic substitution reactions can initiate halogenated disinfection byproduct (DBP) formation. The rate-controlling step (RCS) of electrophilic aromatic halogenation is commonly assumed to be halogen addition (vs proton removal), although this assumption has not been previously assessed under water disinfection conditions. Herein, the herbicide dimethenamid (DM) was used as a model aromatic compound to examine the RCS of halogenation in water treated with free chlorine (chlorination), free chlorine and bromide (bromination), or monochloramine and iodide (iodination). Hydrogen–deuterium kinetic isotope effects (KIEs) were determined using DM (kH) and deuterated DM (kD) in separate reactors. DM chlorination and bromination exhibited no KIE (kH/kD ≈ 1), supporting halogen addition as the RCS. Iodination did, however, exhibit primary KIEs (kH/kD > 1.4), demonstrating some degree of proton removal in the RCS. Increasing the pH (5.2–8.9) and I– concentration (10–100 μM) suppressed iodination rates but did not significantly affect KIEs (1.47–2.06), suggesting that iodine addition is rate-controlling under these conditions ([Cl–] = 15 μM). In contrast, adding 25–100 mM Cl– increased the KIE in iodination (2.85–3.42), reflecting a shift in kinetic control toward proton removal. This study delineates how chloride can both enhance and inhibit electrophilic aromatic iodination, which has implications for I-DBP formation in halide-rich water.
中文翻译:

氯胺化水中丁二烯酰胺的亲电芳香卤代反应中的动力学同位素效应证明了碘化的独特方面
亲电芳香取代反应可引发卤化消毒副产物(DBP)的形成。亲电芳族卤化的速率控制步骤(RCS)通常假定为卤素加成(相对于质子去除),尽管该假设以前未在水消毒条件下进行过评估。在此,将除草剂二甲萘胺(DM)用作模型芳香族化合物,以研究用游离氯(氯化),游离氯和溴化物(溴化)或单氯胺和碘化物(碘化)处理的水中卤化的RCS。使用DM(k H)和氘代DM(k D)在独立的反应堆中确定氢-氘动力学同位素效应(KIEs)。DM氯化和溴化没有KIE(ķ ħ / ķ d ≈1),支撑卤素加成作为RCS。然而,碘化作用确实表现出主要的KIE(k H / k D > 1.4),表明RCS中有一定程度的质子去除。增加pH(5.2–8.9)和I –浓度(10–100μM)抑制了碘化速率,但并未显着影响KIE(1.47–2.06),表明在这些条件下碘的添加是速率控制的[[Cl – ] = 15μM)。相反,添加25–100 mM Cl –增加了碘化的KIE(2.85-3.42),反映了动力学控制向质子去除的转变。这项研究描述了氯化物如何同时增强和抑制亲电子碘化,这对富含卤化物的水中I-DBP的形成有影响。
更新日期:2020-10-13
中文翻译:

氯胺化水中丁二烯酰胺的亲电芳香卤代反应中的动力学同位素效应证明了碘化的独特方面
亲电芳香取代反应可引发卤化消毒副产物(DBP)的形成。亲电芳族卤化的速率控制步骤(RCS)通常假定为卤素加成(相对于质子去除),尽管该假设以前未在水消毒条件下进行过评估。在此,将除草剂二甲萘胺(DM)用作模型芳香族化合物,以研究用游离氯(氯化),游离氯和溴化物(溴化)或单氯胺和碘化物(碘化)处理的水中卤化的RCS。使用DM(k H)和氘代DM(k D)在独立的反应堆中确定氢-氘动力学同位素效应(KIEs)。DM氯化和溴化没有KIE(ķ ħ / ķ d ≈1),支撑卤素加成作为RCS。然而,碘化作用确实表现出主要的KIE(k H / k D > 1.4),表明RCS中有一定程度的质子去除。增加pH(5.2–8.9)和I –浓度(10–100μM)抑制了碘化速率,但并未显着影响KIE(1.47–2.06),表明在这些条件下碘的添加是速率控制的[[Cl – ] = 15μM)。相反,添加25–100 mM Cl –增加了碘化的KIE(2.85-3.42),反映了动力学控制向质子去除的转变。这项研究描述了氯化物如何同时增强和抑制亲电子碘化,这对富含卤化物的水中I-DBP的形成有影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号