当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optogenetic Control of Heterologous Metabolism in E. coli.
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-08-05 , DOI: 10.1021/acssynbio.9b00454 Adhithi R Raghavan 1 , Kevin Salim 1 , Vikramaditya G Yadav 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-08-05 , DOI: 10.1021/acssynbio.9b00454 Adhithi R Raghavan 1 , Kevin Salim 1 , Vikramaditya G Yadav 1
Affiliation
|
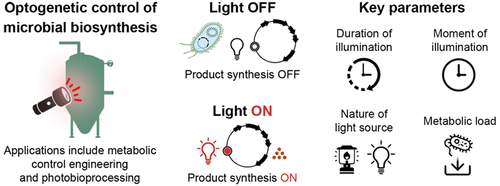
Multiobjective optimization of microbial chassis for the production of xenobiotic compounds requires the implementation of metabolic control strategies that permit dynamic distribution of cellular resources between biomass and product formation. We addressed this need in a previous study by engineering the T7 RNA polymerase to be thermally responsive. The modified polymerase is activated only after the temperature of the host cell falls below 18 °C, and Escherichia coli cells that employ the protein to transcribe the heterologous lycopene biosynthetic pathway exhibit impressive improvements in productivity. We have expanded our toolbox of metabolic switches in the current study by engineering a version of the T7 RNA polymerase that drives the transition between biomass and product formation upon stimulation with red light. The engineered polymerase is expressed as two distinct polypeptide chains. Each chain comprises one of two photoactive components from Arabidopsis thaliana, phytochrome B (PhyB) and phytochrome-integrating factor 3 (PIF3), as well as the N- or C-terminus domains of both, the vacuolar ATPase subunit (VMA) intein of Saccharomyces cerevisiae and the polymerase. Red light drives photodimerization of PhyB and PIF3, which then brings together the N- and C-terminus domains of the VMA intein. Trans-splicing of the intein follows suit and produces an active form of the polymerase that subsequently transcribes any sequence that is under the control of a T7 promoter. The photodimerization also involves a third element, the cyanobacterial chromophore phycocyanobilin (PCB), which too is expressed heterologously by E. coli. We deployed this version of the T7 RNA polymerase to control the production of lycopene in E. coli and observed tight control of pathway expression. We tested a variety of expression configurations to identify one that imposes the lowest metabolic burden on the strain, and we subsequently optimized key parameters such as the source, moment, and duration of photostimulation. We also identified targets for future refinement of the circuit. In summary, our work is a significant advance for the field and greatly expands on previous work by other groups that have used optogenetic circuits to control heterologous metabolism in prokaryotic hosts.
中文翻译:

大肠杆菌异源代谢的光遗传学控制。
用于生产异生物质化合物的微生物底盘的多目标优化需要实施代谢控制策略,允许在生物质和产品形成之间动态分配细胞资源。我们在之前的一项研究中通过将 T7 RNA 聚合酶设计为具有热响应性来满足这一需求。修饰的聚合酶只有在宿主细胞温度低于 18°C 后才会被激活,大肠杆菌使用该蛋白质转录异源番茄红素生物合成途径的细胞表现出令人印象深刻的生产力提高。我们通过设计一种 T7 RNA 聚合酶,在红光刺激下驱动生物量和产物形成之间的转变,从而扩展了当前研究中的代谢开关工具箱。工程聚合酶表达为两条不同的多肽链。每条链包含来自拟南芥的两种光敏成分之一,光敏色素 B (PhyB) 和光敏色素整合因子 3 (PIF3),以及两者的 N 或 C 末端结构域,即液泡 ATP 酶亚基 (VMA) 内含蛋白酿酒酵母和聚合酶。红光驱动 PhyB 和 PIF3 的光二聚化,然后将 VMA 内含肽的 N 端和 C 端结构域结合在一起。内含肽的反式剪接紧随其后,产生聚合酶的活性形式,该聚合酶随后转录受 T7 启动子控制的任何序列。光二聚化还涉及第三种元素,即蓝藻发色团藻蓝胆素 (PCB),它也由大肠杆菌异源表达。我们部署了这个版本的 T7 RNA 聚合酶来控制大肠杆菌中番茄红素的产生并观察到通路表达的严格控制。我们测试了多种表达配置,以确定对菌株施加最低代谢负担的一种,然后我们优化了关键参数,例如光刺激的来源、时刻和持续时间。我们还确定了电路未来改进的目标。总而言之,我们的工作是该领域的重大进步,并且大大扩展了其他团队之前使用光遗传电路控制原核宿主异源代谢的工作。
更新日期:2020-09-20
中文翻译:

大肠杆菌异源代谢的光遗传学控制。
用于生产异生物质化合物的微生物底盘的多目标优化需要实施代谢控制策略,允许在生物质和产品形成之间动态分配细胞资源。我们在之前的一项研究中通过将 T7 RNA 聚合酶设计为具有热响应性来满足这一需求。修饰的聚合酶只有在宿主细胞温度低于 18°C 后才会被激活,大肠杆菌使用该蛋白质转录异源番茄红素生物合成途径的细胞表现出令人印象深刻的生产力提高。我们通过设计一种 T7 RNA 聚合酶,在红光刺激下驱动生物量和产物形成之间的转变,从而扩展了当前研究中的代谢开关工具箱。工程聚合酶表达为两条不同的多肽链。每条链包含来自拟南芥的两种光敏成分之一,光敏色素 B (PhyB) 和光敏色素整合因子 3 (PIF3),以及两者的 N 或 C 末端结构域,即液泡 ATP 酶亚基 (VMA) 内含蛋白酿酒酵母和聚合酶。红光驱动 PhyB 和 PIF3 的光二聚化,然后将 VMA 内含肽的 N 端和 C 端结构域结合在一起。内含肽的反式剪接紧随其后,产生聚合酶的活性形式,该聚合酶随后转录受 T7 启动子控制的任何序列。光二聚化还涉及第三种元素,即蓝藻发色团藻蓝胆素 (PCB),它也由大肠杆菌异源表达。我们部署了这个版本的 T7 RNA 聚合酶来控制大肠杆菌中番茄红素的产生并观察到通路表达的严格控制。我们测试了多种表达配置,以确定对菌株施加最低代谢负担的一种,然后我们优化了关键参数,例如光刺激的来源、时刻和持续时间。我们还确定了电路未来改进的目标。总而言之,我们的工作是该领域的重大进步,并且大大扩展了其他团队之前使用光遗传电路控制原核宿主异源代谢的工作。











































 京公网安备 11010802027423号
京公网安备 11010802027423号