当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Heterocycles by isothiourea organocatalysis
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-08-06 , DOI: 10.1002/jhet.4119 Anup Biswas 1 , Haripriyo Mondal 2 , Modhu Sudan Maji 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-08-06 , DOI: 10.1002/jhet.4119 Anup Biswas 1 , Haripriyo Mondal 2 , Modhu Sudan Maji 2
Affiliation
|
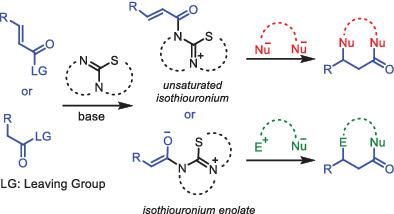
Isothiourea was first employed as catalyst by Birman in 2006 for the enantioselective acyl transfer reaction. The catalyst was then well explored in the course of kinetic resolution and desymmetrization studies. A few years later, Romo and Smith applied isothiourea catalysis in enantioselective cascade reactions to prepare carbocycles and heterocycles acessing new reactivities of isothiourea. Several research groups were then attracted toward this new field of organocatalysis, and applied isothioureas as nucleophilic catalysts in executing cascade methodologies to synthesize various intresteting molecular scaffolds including heterocycles. The present review documents a summary on the construction of heterocyclic molecules by isothiourea organocatalysis. Heterocycles are of prime interest to organic chemists due to their omnipresence in natural products and bioactive molecules. The Lewis basic nucleophilic catalyst isothioureas play a pivotal role in the cascades to generate either α,β‐unsaturated acyl isothiouronium ion or isothiouronium enolate as the prime reaction intermediate. We have covered the reactions involving two intermediates of opposite reactivities affording various heterocycles.
中文翻译:

异硫脲有机催化合成杂环
异硫脲是Birman在2006年首次用于对映选择性酰基转移反应的催化剂。然后在动力学拆分和去对称化研究过程中对催化剂进行了充分的研究。几年后,罗莫和史密斯将异硫脲催化应用于对映选择性级联反应中,以制备具有新异硫脲反应性的碳环和杂环。然后,几个研究小组被吸引到这个新的有机催化领域,并将异硫脲用作亲核催化剂,用于执行级联方法以合成包括链环在内的各种解释性分子支架。本文综述了异硫脲有机催化杂环分子的构建。杂环是有机化学家最感兴趣的化合物,因为它们在天然产物和生物活性分子中无处不在。Lewis碱性亲核催化剂异硫脲在级联反应中产生关键作用α,β-不饱和酰基异硫脲鎓离子或异硫脲鎓烯醇化物为主要反应中间体。我们已经涵盖了涉及两个反应性相反的中间体的反应,这些中间体提供了各种杂环。
更新日期:2020-08-06
中文翻译:

异硫脲有机催化合成杂环
异硫脲是Birman在2006年首次用于对映选择性酰基转移反应的催化剂。然后在动力学拆分和去对称化研究过程中对催化剂进行了充分的研究。几年后,罗莫和史密斯将异硫脲催化应用于对映选择性级联反应中,以制备具有新异硫脲反应性的碳环和杂环。然后,几个研究小组被吸引到这个新的有机催化领域,并将异硫脲用作亲核催化剂,用于执行级联方法以合成包括链环在内的各种解释性分子支架。本文综述了异硫脲有机催化杂环分子的构建。杂环是有机化学家最感兴趣的化合物,因为它们在天然产物和生物活性分子中无处不在。Lewis碱性亲核催化剂异硫脲在级联反应中产生关键作用α,β-不饱和酰基异硫脲鎓离子或异硫脲鎓烯醇化物为主要反应中间体。我们已经涵盖了涉及两个反应性相反的中间体的反应,这些中间体提供了各种杂环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号