当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and bioactivities of novel pyridazinone derivatives containing 2‐phenylthiazole or oxazole skeletons
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-08-05 , DOI: 10.1002/jhet.4118 Mingming Dang 1, 2 , Minhua Liu 2, 3 , Lu Huang 2, 4 , Xiaoming Ou 2, 4 , Chuyun Long 2, 4 , Xingping Liu 2, 3 , Yeguo Ren 2, 3 , Ping Zhang 2, 4 , Mingzhi Huang 2, 3 , Aiping Liu 2, 3
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-08-05 , DOI: 10.1002/jhet.4118 Mingming Dang 1, 2 , Minhua Liu 2, 3 , Lu Huang 2, 4 , Xiaoming Ou 2, 4 , Chuyun Long 2, 4 , Xingping Liu 2, 3 , Yeguo Ren 2, 3 , Ping Zhang 2, 4 , Mingzhi Huang 2, 3 , Aiping Liu 2, 3
Affiliation
|
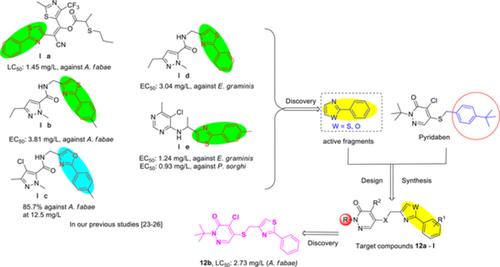
A series of novel pyridazinone derivatives were designed and synthesized by replacing 4‐(tert‐butyl)phenyl moiety of pyridaben with 2‐phenylthiazole or oxazole fragments via activity substructure connecting approach. The structures of all target compounds were characterized through NMR, MS, and elemental analysis. Bioassay results exhibit that most compounds showed potent bioactivities against Aphis fabae, Tetranychus urticae, Erysiphe graminis, and/or Puccinia polysora. Among the newly synthesized compounds, 2‐(tert‐butyl)‐4‐chloro‐5‐(((2‐phenylthiazol‐4‐yl)methyl)thio)pyridazin‐3(2H)‐one (12b) displays remarkable insecticidal activity against A fabae. Its LC50 value (2.73 mg/L) is better than that of pyridaben (5.46 mg/L), although inferior to that of imidacloprid (0.51 mg/L). In addition to its extraordinary insecticidal activity, compound 12b also exerts 96.9% fungicidal activities against P polysora at 500 mg/L in vivo, significantly superior to that of pyridaben (50.0%), while slightly lower than that of tebuconazole (100%). This article discusses the synthesis, bioassay results, and structure‐activity relationship of this series of novel pyridazinone derivatives.
中文翻译:

新型含2-苯基噻唑或恶唑骨架的哒嗪酮衍生物的设计,合成和生物活性
通过活性亚结构连接方法,用2-苯基噻唑或恶唑片段取代了哒嗪的4-(叔丁基)苯基部分,设计并合成了一系列新型的哒嗪酮衍生物。所有目标化合物的结构均通过NMR,MS和元素分析表征。生物测定的结果表明,大多数化合物显示出对Fabs,Tetranychus urticae,Erysiphe graminis和/或Puccinia polysora的有效生物活性。在新合成的化合物中,2-(叔丁基)-4-氯5-((((2-苯基噻唑-4-基)甲基)硫基)哒嗪-3(2 H)-one(12b)对Fab表现出明显的杀虫活性。它的LC 50值(2.73 mg / L)比吡虫本(5.46 mg / L)好,尽管比吡虫啉(0.51 mg / L)低。除具有非凡的杀虫活性外,化合物12b在体内500 mg / L时对多聚磷也具有96.9%的杀真菌活性,显着优于吡达本(50.0%),但略低于戊唑醇(100%)。本文讨论了该系列新型哒嗪酮衍生物的合成,生物测定结果和构效关系。
更新日期:2020-08-05
中文翻译:

新型含2-苯基噻唑或恶唑骨架的哒嗪酮衍生物的设计,合成和生物活性
通过活性亚结构连接方法,用2-苯基噻唑或恶唑片段取代了哒嗪的4-(叔丁基)苯基部分,设计并合成了一系列新型的哒嗪酮衍生物。所有目标化合物的结构均通过NMR,MS和元素分析表征。生物测定的结果表明,大多数化合物显示出对Fabs,Tetranychus urticae,Erysiphe graminis和/或Puccinia polysora的有效生物活性。在新合成的化合物中,2-(叔丁基)-4-氯5-((((2-苯基噻唑-4-基)甲基)硫基)哒嗪-3(2 H)-one(12b)对Fab表现出明显的杀虫活性。它的LC 50值(2.73 mg / L)比吡虫本(5.46 mg / L)好,尽管比吡虫啉(0.51 mg / L)低。除具有非凡的杀虫活性外,化合物12b在体内500 mg / L时对多聚磷也具有96.9%的杀真菌活性,显着优于吡达本(50.0%),但略低于戊唑醇(100%)。本文讨论了该系列新型哒嗪酮衍生物的合成,生物测定结果和构效关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号