当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
RAB39B's role in membrane traffic, autophagy, and associated neuropathology.
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-08-06 , DOI: 10.1002/jcp.29962 Bor Luen Tang 1, 2
Journal of Cellular Physiology ( IF 5.6 ) Pub Date : 2020-08-06 , DOI: 10.1002/jcp.29962 Bor Luen Tang 1, 2
Affiliation
|
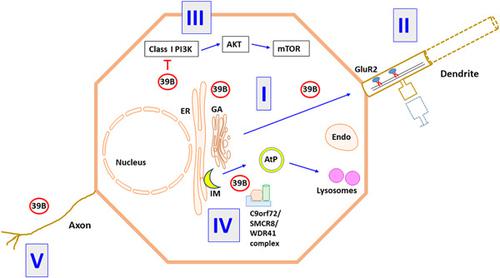
Neuropathological disorders are increasingly associated with dysfunctions in neuronal membrane traffic and autophagy, with defects among members of the Rab family of small GTPases implicated. Mutations in the human Xq28 localized gene RAB39B have been associated with X‐linked neurodevelopmental defects including macrocephaly, intellectual disability, autism spectrum disorder (ASD), as well as rare cases of early‐onset Parkinson's disease (PD). Despite the finding that RAB39B regulates GluA2 trafficking and could thus influence synaptic α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor subunit composition, reasons for the wide‐ranging neuropathological consequences associated with RAB39B defects have been unclear. Recent studies have now unraveled possible mechanisms underlying the neuropathological roles of this brain‐enriched small GTPase. Studies in RAB39B knockout mice showed that RAB39B interacts with components of Class I phosphatidylinositol‐3‐kinase (PI3K) signaling. In its absence, the PI3K‐AKT‐mechanistic target of rapamycin signaling pathway in neural progenitor cells (NPCs) is hyperactivated, which promotes NPC proliferation, leading to macrocephaly and ASD. Pertaining to early‐onset PD, a complex of C9orf72, Smith–Magenis syndrome chromosome region candidate 8 and WD repeat domain 41 that functions in autophagy has been identified as a guanine nucleotide exchange factor of RAB39B. Here, recent findings that have shed light on our mechanistic understanding of RAB39B's role in neurodevelopmental and neurodegenerative pathologies are reviewed. Caveats and unanswered questions are also discussed, and future perspectives outlined.
中文翻译:

RAB39B 在膜运输、自噬和相关神经病理学中的作用。
神经病理学疾病越来越多地与神经元膜运输和自噬功能障碍相关,其中涉及小 GTP 酶 Rab 家族成员的缺陷。人类 Xq28 定位基因RAB39B 的突变与 X 连锁神经发育缺陷有关,包括大头畸形、智力障碍、自闭症谱系障碍 (ASD) 以及罕见的早发性帕金森病 (PD)。尽管发现 RAB39B 调节 GluA2 运输并因此可能影响突触 α-氨基-3-羟基-5-甲基-4-异恶唑丙酸受体亚基组成,但与 RAB39B 缺陷相关的广泛神经病理学后果的原因尚不清楚。最近的研究现已揭示了这种富含大脑的小 GTPase 的神经病理学作用的可能机制。在 RAB39B 基因敲除小鼠中的研究表明,RAB39B 与 I 类磷脂酰肌醇 3-激酶 (PI3K) 信号传导的成分相互作用。在它缺席的情况下,神经祖细胞 (NPC) 中雷帕霉素信号通路的 PI3K-AKT 机制靶标过度激活,促进 NPC 增殖,导致巨头畸形和 ASD。关于早发性 PD,C9orf72、Smith-Magenis 综合征候选染色体区域 8 和 WD 重复结构域 41 的复合物在自噬中起作用,已被鉴定为 RAB39B 的鸟嘌呤核苷酸交换因子。在这里,回顾了最近的发现,这些发现阐明了我们对 RAB39B 在神经发育和神经退行性疾病中的作用的机制理解。还讨论了注意事项和未解决的问题,并概述了未来的前景。Smith-Magenis 综合征染色体候选区域 8 和在自噬中起作用的 WD 重复结构域 41 已被鉴定为 RAB39B 的鸟嘌呤核苷酸交换因子。在这里,回顾了最近的发现,这些发现阐明了我们对 RAB39B 在神经发育和神经退行性疾病中的作用的机制理解。还讨论了注意事项和未解决的问题,并概述了未来的前景。Smith-Magenis 综合征染色体候选区域 8 和在自噬中起作用的 WD 重复结构域 41 已被鉴定为 RAB39B 的鸟嘌呤核苷酸交换因子。在这里,回顾了最近的发现,这些发现阐明了我们对 RAB39B 在神经发育和神经退行性疾病中的作用的机制理解。还讨论了注意事项和未解决的问题,并概述了未来的前景。
更新日期:2020-08-06
中文翻译:

RAB39B 在膜运输、自噬和相关神经病理学中的作用。
神经病理学疾病越来越多地与神经元膜运输和自噬功能障碍相关,其中涉及小 GTP 酶 Rab 家族成员的缺陷。人类 Xq28 定位基因RAB39B 的突变与 X 连锁神经发育缺陷有关,包括大头畸形、智力障碍、自闭症谱系障碍 (ASD) 以及罕见的早发性帕金森病 (PD)。尽管发现 RAB39B 调节 GluA2 运输并因此可能影响突触 α-氨基-3-羟基-5-甲基-4-异恶唑丙酸受体亚基组成,但与 RAB39B 缺陷相关的广泛神经病理学后果的原因尚不清楚。最近的研究现已揭示了这种富含大脑的小 GTPase 的神经病理学作用的可能机制。在 RAB39B 基因敲除小鼠中的研究表明,RAB39B 与 I 类磷脂酰肌醇 3-激酶 (PI3K) 信号传导的成分相互作用。在它缺席的情况下,神经祖细胞 (NPC) 中雷帕霉素信号通路的 PI3K-AKT 机制靶标过度激活,促进 NPC 增殖,导致巨头畸形和 ASD。关于早发性 PD,C9orf72、Smith-Magenis 综合征候选染色体区域 8 和 WD 重复结构域 41 的复合物在自噬中起作用,已被鉴定为 RAB39B 的鸟嘌呤核苷酸交换因子。在这里,回顾了最近的发现,这些发现阐明了我们对 RAB39B 在神经发育和神经退行性疾病中的作用的机制理解。还讨论了注意事项和未解决的问题,并概述了未来的前景。Smith-Magenis 综合征染色体候选区域 8 和在自噬中起作用的 WD 重复结构域 41 已被鉴定为 RAB39B 的鸟嘌呤核苷酸交换因子。在这里,回顾了最近的发现,这些发现阐明了我们对 RAB39B 在神经发育和神经退行性疾病中的作用的机制理解。还讨论了注意事项和未解决的问题,并概述了未来的前景。Smith-Magenis 综合征染色体候选区域 8 和在自噬中起作用的 WD 重复结构域 41 已被鉴定为 RAB39B 的鸟嘌呤核苷酸交换因子。在这里,回顾了最近的发现,这些发现阐明了我们对 RAB39B 在神经发育和神经退行性疾病中的作用的机制理解。还讨论了注意事项和未解决的问题,并概述了未来的前景。



























 京公网安备 11010802027423号
京公网安备 11010802027423号