当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of HEC72702 chirality on the selective inhibition of hepatitis B virus capsid dimer: A dynamics-structure-energetics perspective.
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-08-05 , DOI: 10.1111/cbdd.13771 Abdolkarim Farrokhzadeh 1 , Farideh Badichi Akher 2, 3 , Fisayo A Olotu 4 , Fanie R Van Heerden 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2020-08-05 , DOI: 10.1111/cbdd.13771 Abdolkarim Farrokhzadeh 1 , Farideh Badichi Akher 2, 3 , Fisayo A Olotu 4 , Fanie R Van Heerden 1
Affiliation
|
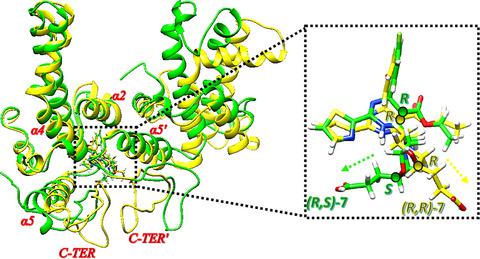
Chirality in drug design has been attracting wide interests and attention over the years based on its innate potentials of enhancing the selectivity and prowess of therapeutic molecules. This approach was fundamental to the recent design of two inhibitors, where (R,R)‐HEC72702 exhibited higher potency inhibition against hepatitis B virus capsid (HBVC) than (R,S)‐HEC72702. Nevertheless, the detailed molecular mechanism has remained unresolved. Here, we apply multiple computational approaches to explore, validate, and differentiate the binding modes of (R,R) and (R,S)‐HEC72702 and to explain the systematic roles mediated by chirality on the distinctive inhibition of HBVC dimer (HBVCd). Our findings revealed that chirality change from R,S to R,R engenders variations in the position of the propanoic acid group of HEC72702 toward the α5′ and C‐TER′ region of HBVCd chain B which could explain the higher inhibitory affinity of (R,R)‐HEC72702. Estimated binding free energies revealed a good correlation with bioactivity data. Moreover, analysis of energy decomposition revealed the prominent effects of van der Waals interactions in the binding process of both compounds to HBVCd. Furthermore, hierarchical clustering of residue‐based energetic contributions suggested two hot‐spot residues W125´ and F156´ play crucial roles in the systematic motions of the propanoic acid group toward chain B.
中文翻译:

HEC72702 手性对乙型肝炎病毒衣壳二聚体选择性抑制的影响:动力学-结构-能量学视角。
多年来,药物设计中的手性因其增强治疗分子的选择性和能力的先天潜力而引起了广泛的兴趣和关注。这种方法是最近设计的两种抑制剂的基础,其中(R,R )-HEC72702 对乙型肝炎病毒衣壳 (HBVC) 的抑制效力高于 ( R,S )-HEC72702。然而,详细的分子机制仍未得到解决。在这里,我们应用多种计算方法来探索、验证和区分 ( R,R ) 和 ( R,S )的结合模式-HEC72702 并解释手性介导的系统作用对 HBVC 二聚体 (HBVCd) 的独特抑制。我们的研究结果表明从手性变化R,S对- [R ,- [R在朝向α5'和C-TER'HBVCd链B的区域HEC72702的丙酸基团的位置滋生的变化,这可以解释(的更高的抑制亲和力ř , R ) -HEC72702。估计的结合自由能显示出与生物活性数据的良好相关性。此外,能量分解分析揭示了范德华相互作用在两种化合物与 HBVCd 结合过程中的显着影响。此外,基于残基的能量贡献的层次聚类表明,两个热点残基 W125´ 和 F156´ 在丙酸基团向 B 链的系统运动中起关键作用。
更新日期:2020-08-05
中文翻译:

HEC72702 手性对乙型肝炎病毒衣壳二聚体选择性抑制的影响:动力学-结构-能量学视角。
多年来,药物设计中的手性因其增强治疗分子的选择性和能力的先天潜力而引起了广泛的兴趣和关注。这种方法是最近设计的两种抑制剂的基础,其中(R,R )-HEC72702 对乙型肝炎病毒衣壳 (HBVC) 的抑制效力高于 ( R,S )-HEC72702。然而,详细的分子机制仍未得到解决。在这里,我们应用多种计算方法来探索、验证和区分 ( R,R ) 和 ( R,S )的结合模式-HEC72702 并解释手性介导的系统作用对 HBVC 二聚体 (HBVCd) 的独特抑制。我们的研究结果表明从手性变化R,S对- [R ,- [R在朝向α5'和C-TER'HBVCd链B的区域HEC72702的丙酸基团的位置滋生的变化,这可以解释(的更高的抑制亲和力ř , R ) -HEC72702。估计的结合自由能显示出与生物活性数据的良好相关性。此外,能量分解分析揭示了范德华相互作用在两种化合物与 HBVCd 结合过程中的显着影响。此外,基于残基的能量贡献的层次聚类表明,两个热点残基 W125´ 和 F156´ 在丙酸基团向 B 链的系统运动中起关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号