当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereospecific Reactions Leading to Allylboronic Esters Within Acyclic Systems Bearing Distant Stereocenters.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-05 , DOI: 10.1002/anie.202010135 David Pierrot 1 , Ilan Marek 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-05 , DOI: 10.1002/anie.202010135 David Pierrot 1 , Ilan Marek 1
Affiliation
|
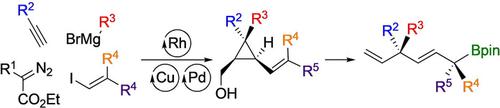
The preparation of acyclic molecules featuring congested stereocenters in a 1,4‐relationship in only three catalytic steps from commercially available building blocks is reported. This approach involves a diastereoselective diboration of alkenyl cyclopropyl methanol derivatives followed by a regioselective exergonic ring fragmentation. The starting materials can be prepared enantiomerically enriched and all substituents can be interconverted, therefore, this strategy allows a large variety of diversely functionalized allylboronic esters possessing distant tetrasubstituted stereocenters with high diastereoselectivity.
中文翻译:

立体异构反应导致在带有遥远立体中心的无环体系中产生烯丙基硼酸酯。
据报道,仅在三个催化步骤中,就可以从市售的构建基块中以1,4关系形成具有拥挤的立体中心的无环分子。该方法涉及烯基环丙基甲醇衍生物的非对映选择性二硼化,然后进行区域选择性能拓环断裂。可以制备对映体富集的起始原料,并且所有取代基都可以相互转化,因此,该策略允许具有高非对映选择性的,具有遥远的四取代立体中心的多种多样的官能化烯丙基硼酸酯。
更新日期:2020-08-05
中文翻译:

立体异构反应导致在带有遥远立体中心的无环体系中产生烯丙基硼酸酯。
据报道,仅在三个催化步骤中,就可以从市售的构建基块中以1,4关系形成具有拥挤的立体中心的无环分子。该方法涉及烯基环丙基甲醇衍生物的非对映选择性二硼化,然后进行区域选择性能拓环断裂。可以制备对映体富集的起始原料,并且所有取代基都可以相互转化,因此,该策略允许具有高非对映选择性的,具有遥远的四取代立体中心的多种多样的官能化烯丙基硼酸酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号