Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2020-08-06 , DOI: 10.1016/j.jphotochem.2020.112830 Prakash Majee , Pooja Daga , Debal Kanti Singha , Debabrata Saha , Partha Mahata , Sudip Kumar Mondal
|
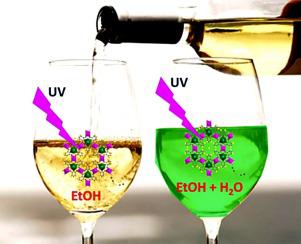
It is very crucial to have a very simple, instant and low-cost detection and estimation of a trace of water in common organic solvents including alcohols. To obtain the sensor material we have synthesized a metal-organic framework (MOF) {[Y1.0Mn1.5(PDA)3(H2O)3]·3.5H2O}, 1, (PDA = 2,6-pyridinedicarboxylic acid) through hydrothermal process. A doping of compound 1 by 10 % terbium {[Y0.9Tb0.1Mn1.5(PDA)3(H2O)3]·3.5H2O}, 1:Tb, was done through an isomorphous substitution technique. The most advantageous point about 1:Tb was the metal centre luminescence, which was largely stokes shifted from the excitation light making possible the naked eye observation. The weak metal centre luminescence intensity of dehydrated 1:Tb (excluding lattice and coordinated water molecules) in organic solvents EtOH, CH3OH, CH3CN, THF and n-heptane showed huge turn-on in presence of trace amounts of H2O in the said solvents. The luminescence intensity of Tb3+ centre was enhanced by several folds with a limit of detection 1.12 %(v/v), 0.47 %(v/v), 0.04 %(v/v), 0.13 %(v/v) and 0.53 %(v/v) respectively. The coordinated water molecules as well as the lattice water molecules in 1:Tb play a vital role during sensitization of the Tb3+ centre by enhancing the rigidity of the structure and facilitating the formation of LMCT state which ultimately results in a huge turn-on of metal centre luminescence.
中文翻译:

掺杂镧系元素的金属有机框架通过肉眼检测器通过监测发光的开启而证明了在有机溶剂(包括醇)中的痕量水
对包括酒精在内的普通有机溶剂中的痕量水进行非常简单,即时和低成本的检测和估算,这一点至关重要。为了获得传感器材料,我们合成了金属有机骨架(MOF){[Y 1.0 Mn 1.5(PDA)3(H 2 O)3 ]·3.5H 2 O},1((PDA = 2,6-吡啶二羧酸酸)通过水热过程。用10%{{[Y 0.9 Tb 0.1 Mn 1.5(PDA)3(H 2 O)3 ]·3.5H 2 O},1:Tb掺杂化合物1,是通过同构取代技术完成的。大约1:Tb的最有利点是金属中心发光,它与激发光的斯托克斯偏移很大,因此可以用肉眼观察。脱水的弱金属中心的发光强度1:TB(不含晶格和配位水分子)在有机溶剂中的EtOH,CH 3 OH,CH 3 CN,THF和正庚烷表明巨大的接通中的h的痕量存在2在上述溶剂中为O。Tb 3+的发光强度中心增强了几倍,检出限为1.12%(v / v),0.47%(v / v),0.04%(v / v),0.13%(v / v)和0.53%(v / v)分别。1:Tb中的配位水分子和晶格水分子在Tb 3+中心敏化过程中起着至关重要的作用,它增强了结构的刚性并促进了LMCT状态的形成,最终导致了巨大的开启金属中心发光。











































 京公网安备 11010802027423号
京公网安备 11010802027423号