当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of interaction between tea polyphenols with soymilk protein on inactivation of soybean trypsin inhibitor
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.foodhyd.2020.106177 Ge Ge , Wanxiang Guo , Jiabao Zheng , Mouming Zhao , Weizheng Sun
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.foodhyd.2020.106177 Ge Ge , Wanxiang Guo , Jiabao Zheng , Mouming Zhao , Weizheng Sun
|
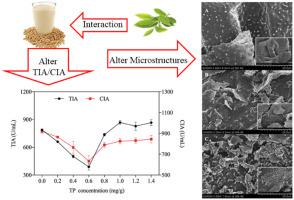
Abstract Soymilk is a popular health beverage worldwide, but its nutritional value is limited by soybean trypsin inhibitors (STIs). The interaction of tea polyphenols (TPs) with soymilk protein in the complex system was investigated in this work. TPs interacted with soymilk protein via static quenching process and hydrophobic interaction, with binding constant (Ka) of 5.22 × 103 L mol−1 at 298 K. Synchronous fluorescence suggested that the binding site of TPs to soymilk protein was mainly at Trp residues compared with Tyr residues. FTIR analysis revealed that hydrogen bonds were also observed in TPs-soymilk system. CD spectroscopy suggested that the protein conformation became more stable with addition of TPs by reducing β-sheet and random coil, and increasing α-helix and β-turn. The trypsin and chymotrypsin inhibitory activities (TIA and CIA) were reduced at 0.6 mg/g TPs in cooked soymilk from 788.3 ± 10.4 U/mL and 918.7 ± 18.0 U/mL to 388.3 ± 35.5 U/mL and 633.3 ± 52.8 U/mL, respectively.
中文翻译:

茶多酚与豆浆蛋白相互作用对大豆胰蛋白酶抑制剂失活的影响
摘要 豆浆是一种全球流行的健康饮料,但其营养价值受到大豆胰蛋白酶抑制剂(STI)的限制。在这项工作中研究了复杂系统中茶多酚 (TPs) 与豆浆蛋白的相互作用。TPs通过静态猝灭和疏水相互作用与豆浆蛋白相互作用,298 K时结合常数(Ka)为5.22×103 L mol-1。同步荧光表明TPs与豆浆蛋白的结合位点主要在Trp残基上。酪氨酸残基。FTIR 分析表明,在 TPs-豆浆体系中也观察到氢键。CD光谱表明,通过减少β-折叠和无规卷曲以及增加α-螺旋和β-转角,蛋白质构象随着TP的加入而变得更加稳定。
更新日期:2021-02-01
中文翻译:

茶多酚与豆浆蛋白相互作用对大豆胰蛋白酶抑制剂失活的影响
摘要 豆浆是一种全球流行的健康饮料,但其营养价值受到大豆胰蛋白酶抑制剂(STI)的限制。在这项工作中研究了复杂系统中茶多酚 (TPs) 与豆浆蛋白的相互作用。TPs通过静态猝灭和疏水相互作用与豆浆蛋白相互作用,298 K时结合常数(Ka)为5.22×103 L mol-1。同步荧光表明TPs与豆浆蛋白的结合位点主要在Trp残基上。酪氨酸残基。FTIR 分析表明,在 TPs-豆浆体系中也观察到氢键。CD光谱表明,通过减少β-折叠和无规卷曲以及增加α-螺旋和β-转角,蛋白质构象随着TP的加入而变得更加稳定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号