当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interfacial water shuffling the intermediates of hydrogen oxidation and evolution reactions in aqueous media
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-08-05 , DOI: 10.1039/d0ee01754j Ershuai Liu 1, 2, 3, 4 , Li Jiao 2, 3, 4, 5 , Jingkun Li 1, 2, 3, 4 , Thomas Stracensky 1, 2, 3, 4 , Qiang Sun 1, 2, 3, 4 , Sanjeev Mukerjee 1, 2, 3, 4 , Qingying Jia 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-08-05 , DOI: 10.1039/d0ee01754j Ershuai Liu 1, 2, 3, 4 , Li Jiao 2, 3, 4, 5 , Jingkun Li 1, 2, 3, 4 , Thomas Stracensky 1, 2, 3, 4 , Qiang Sun 1, 2, 3, 4 , Sanjeev Mukerjee 1, 2, 3, 4 , Qingying Jia 1, 2, 3, 4
Affiliation
|
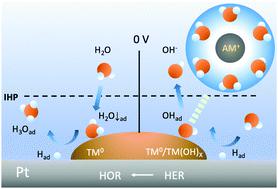
The kinetics of the hydrogen oxidation and evolution reactions (HOR/HER) of platinum in aqueous solutions remains elusive, partly because of the lack of means to explore the surface–electrolyte interface. Herein, we probe this interface by utilizing surface transition metals (TMs), carbon monoxide, alkali metal cations (AM+), and heavy water in combination with in situ X-ray absorption spectroscopy. It was found that the surface TMs in the metallic phase may boost the HOR kinetics of Pt in alkali by hosting the interfacial water with the oxygen-down orientation that removes the adsorbed hydrogen on Pt neighbors. Furthermore, surface TMs in either metallic or hydroxide phases improve the HER kinetics of Pt by hosting the hydroxyl generated from water dissociation so it can be desorbed by the interfacial water coordinated to AM+. The roles of interfacial water in shuffling the HOR/HER intermediates throughout the interface are supported by kinetic isotope effects.
中文翻译:

界面水改组水性介质中氢氧化和放出反应的中间体
水溶液中铂的氢氧化和析出反应(HOR / HER)的动力学仍然难以捉摸,部分原因是缺乏探索表面-电解质界面的方法。在此,我们通过利用表面的过渡金属(TMS),一氧化碳,碱金属阳离子(AM探测该接口+),和重水结合原位X射线吸收光谱法。已经发现,金属相中的表面TMs可以通过以降低氧的取向容纳界面水,从而去除Pt邻域上吸附的氢,从而提高碱中Pt的HOR动力学。此外,金属相或氢氧化物相中的表面TM通过容纳水分解产生的羟基来改善Pt的HER动力学,因此可以被配位于AM +的界面水解吸。动力学同位素效应支持了界面水在重整整个界面中的HOR / HER中间体中的作用。
更新日期:2020-09-16
中文翻译:

界面水改组水性介质中氢氧化和放出反应的中间体
水溶液中铂的氢氧化和析出反应(HOR / HER)的动力学仍然难以捉摸,部分原因是缺乏探索表面-电解质界面的方法。在此,我们通过利用表面的过渡金属(TMS),一氧化碳,碱金属阳离子(AM探测该接口+),和重水结合原位X射线吸收光谱法。已经发现,金属相中的表面TMs可以通过以降低氧的取向容纳界面水,从而去除Pt邻域上吸附的氢,从而提高碱中Pt的HOR动力学。此外,金属相或氢氧化物相中的表面TM通过容纳水分解产生的羟基来改善Pt的HER动力学,因此可以被配位于AM +的界面水解吸。动力学同位素效应支持了界面水在重整整个界面中的HOR / HER中间体中的作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号