当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How to complete the tautomerization and substrate-assisted activation prior to C–C bond fission by meta-cleavage product hydrolase LigY?
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-08-04 , DOI: 10.1039/d0cy01102a Junjie Wang 1, 2, 3, 4 , Xiaowen Tang 4, 5, 6, 7 , Yixin Zhang 1, 2, 3, 4 , Yanwei Li 1, 2, 3, 4 , Ledong Zhu 1, 2, 3, 4 , Qingzhu Zhang 1, 2, 3, 4 , Wenxing Wang 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-08-04 , DOI: 10.1039/d0cy01102a Junjie Wang 1, 2, 3, 4 , Xiaowen Tang 4, 5, 6, 7 , Yixin Zhang 1, 2, 3, 4 , Yanwei Li 1, 2, 3, 4 , Ledong Zhu 1, 2, 3, 4 , Qingzhu Zhang 1, 2, 3, 4 , Wenxing Wang 1, 2, 3, 4
Affiliation
|
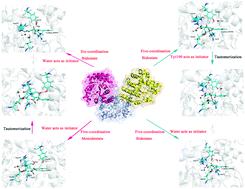
LigY belongs to the amidohydrolase superfamily responsible for the C–C bond fission of meta-cleavage products (MCPs). LigY employs similar nucleophile-activation strategies like previously reported serine-dependent MCP hydrolases; however, the mechanisms of the tautomerization and hydrolysis are different. In this study, the quantum mechanics/molecular mechanics (QM/MM) approach was performed to elucidate the mechanism of MCP C–C bond fission by LigY. The binding (monodentate or bidentate) of the substrate to a zinc ion plays a significant role in the tautomerization. Both binding modes could complete the tautomerization and hydrolysis; however, the monodentate mode and five-coordination might be the most likely binding mode. For the bidentate binding mode, unlike the mechanism proposed based on experiments, Tyr190 acts as a “proton transfer” bridge to complete the tautomerization and indirectly activates the catalytic water. For the monodentate binding mode, interestingly, Tyr190/Arg234* (from the neighboring protomer B) act as a proton donor for the activated deprotonated water required to complete the tautomerization. A strong hydrogen bond is formed in this step to stabilize the water and prevent it from moving away from the substrate (C6-carbonyl), leading to the subsequent C–C bond fragmentation. Furthermore, Arg72 plays a crucial role in the monodentate binding of the substrate to Zn(II). Our work expanded the understanding of the mechanism of MCP C–C bond fragmentation by LigY, especially details of the tautomerization and substrate-assisted activation prior to C–C bond fission.
中文翻译:

在通过元裂解产物水解酶LigY进行C–C键裂变之前,如何完成互变异构和底物辅助活化?
LigY属于酰胺水解酶对的C-C键断裂超家族负责元裂解产品(MCP)。LigY采用类似的亲核试剂活化策略,如先前报道的丝氨酸依赖性MCP水解酶;但是,互变异构和水解的机理是不同的。在这项研究中,进行了量子力学/分子力学(QM / MM)方法,以阐明LigY对MCP C–C键裂变的机理。底物与锌离子的结合(单齿或双齿)在互变异构中起重要作用。两种结合方式均可完成互变异构和水解。但是,单齿模式和五协作可能是最可能的绑定模式。对于双齿结合模式,与基于实验提出的机理不同,Tyr190充当“质子转移”桥来完成互变异构并间接活化催化水。对于单齿结合模式,有趣的是,Tyr190 / Arg234 *(来自邻近的protomer B)充当完成互变异构所需的活化去质子化水的质子供体。在此步骤中形成了牢固的氢键,以稳定水并防止水从基材上移走(C6-羰基),导致随后的C–C键断裂。此外,Arg72在底物与Zn( II)的单齿结合中起关键作用。我们的工作扩大了对LigY对MCP C–C键断裂机理的理解,尤其是有关C–C键裂变之前互变异构和底物辅助活化的细节。
更新日期:2020-09-05
中文翻译:

在通过元裂解产物水解酶LigY进行C–C键裂变之前,如何完成互变异构和底物辅助活化?
LigY属于酰胺水解酶对的C-C键断裂超家族负责元裂解产品(MCP)。LigY采用类似的亲核试剂活化策略,如先前报道的丝氨酸依赖性MCP水解酶;但是,互变异构和水解的机理是不同的。在这项研究中,进行了量子力学/分子力学(QM / MM)方法,以阐明LigY对MCP C–C键裂变的机理。底物与锌离子的结合(单齿或双齿)在互变异构中起重要作用。两种结合方式均可完成互变异构和水解。但是,单齿模式和五协作可能是最可能的绑定模式。对于双齿结合模式,与基于实验提出的机理不同,Tyr190充当“质子转移”桥来完成互变异构并间接活化催化水。对于单齿结合模式,有趣的是,Tyr190 / Arg234 *(来自邻近的protomer B)充当完成互变异构所需的活化去质子化水的质子供体。在此步骤中形成了牢固的氢键,以稳定水并防止水从基材上移走(C6-羰基),导致随后的C–C键断裂。此外,Arg72在底物与Zn( II)的单齿结合中起关键作用。我们的工作扩大了对LigY对MCP C–C键断裂机理的理解,尤其是有关C–C键裂变之前互变异构和底物辅助活化的细节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号