当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Analysis of Binding Determinants of Salmonella typhimurium Trehalose-6-phosphate Phosphatase Using Ground-State Complexes.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-04 , DOI: 10.1021/acs.biochem.0c00317 Christine M Harvey 1 , Katherine H O'Toole 1 , Chunliang Liu 2 , Patrick Mariano 2 , Debra Dunaway-Mariano 2 , Karen N Allen 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-04 , DOI: 10.1021/acs.biochem.0c00317 Christine M Harvey 1 , Katherine H O'Toole 1 , Chunliang Liu 2 , Patrick Mariano 2 , Debra Dunaway-Mariano 2 , Karen N Allen 1
Affiliation
|
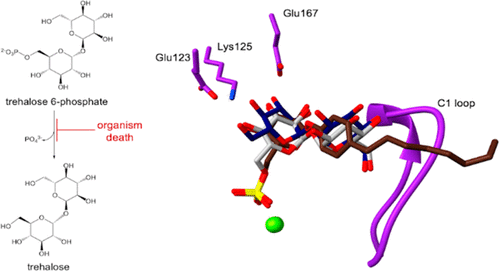
Trehalose-6-phosphate phosphatase (T6PP) catalyzes the dephosphorylation of trehalose 6-phosphate (T6P) to the disaccharide trehalose. The enzyme is not present in mammals but is essential to the viability of multiple lower organisms as trehalose is a critical metabolite, and T6P accumulation is toxic. Hence, T6PP is a target for therapeutics of human pathologies caused by bacteria, fungi, and parasitic nematodes. Here, we report the X-ray crystal structures of Salmonella typhimurium T6PP (StT6PP) in its apo form and in complex with the cofactor Mg2+ and the substrate analogue trehalose 6-sulfate (T6S), the product trehalose, or the competitive inhibitor 4-n-octylphenyl α-d-glucopyranoside 6-sulfate (OGS). OGS replaces the substrate phosphoryl group with a sulfate group and the glucosyl ring distal to the sulfate group with an octylphenyl moiety. The structures of these substrate-analogue and product complexes with T6PP show that specificity is conferred via hydrogen bonds to the glucosyl group proximal to the phosphoryl moiety through Glu123, Lys125, and Glu167, conserved in T6PPs from multiple species. The structure of the first-generation inhibitor OGS shows that it retains the substrate-binding interactions observed for the sulfate group and the proximal glucosyl ring. The OGS octylphenyl moiety binds in a unique manner, indicating that this subsite can tolerate various chemotypes. Together, these findings show that these conserved interactions at the proximal glucosyl ring binding site could provide the basis for the development of broad-spectrum therapeutics, whereas variable interactions at the divergent distal subsite could present an opportunity for the design of potent organism-specific therapeutics.
中文翻译:

使用基态复合物对鼠伤寒沙门氏菌 Trehalose-6-phosphate 磷酸酶的结合决定簇进行结构分析。
6-磷酸海藻糖磷酸酶 (T6PP) 催化 6-磷酸海藻糖 (T6P) 脱磷酸化为二糖海藻糖。该酶不存在于哺乳动物体内,但对多种低等生物的生存至关重要,因为海藻糖是一种关键的代谢物,而 T6P 的积累是有毒的。因此,T6PP 是治疗由细菌、真菌和寄生线虫引起的人类病理学的靶标。在这里,我们报告了载脂蛋白形式的鼠伤寒沙门氏菌T6PP ( St T6PP)以及与辅助因子 Mg 2+和底物类似物海藻糖 6-硫酸盐 (T6S)、产品海藻糖或竞争性复合物的X 射线晶体结构抑制剂 4-正辛基苯基α- d-吡喃葡萄糖苷 6-硫酸盐 (OGS)。OGS 用硫酸基取代底物磷酰基,用辛基苯基部分取代硫酸基远端的葡糖基环。这些具有 T6PP 的底物类似物和产物复合物的结构表明,特异性是通过氢键通过 Glu123、Lys125 和 Glu167 与磷酰基部分近端的葡糖基结合而赋予的,这在来自多个物种的 T6PP 中是保守的。第一代抑制剂 OGS 的结构表明,它保留了硫酸基团和近端糖基环观察到的底物结合相互作用。OGS 辛基苯基部分以独特的方式结合,表明该亚位点可以耐受各种化学型。一起,
更新日期:2020-09-08
中文翻译:

使用基态复合物对鼠伤寒沙门氏菌 Trehalose-6-phosphate 磷酸酶的结合决定簇进行结构分析。
6-磷酸海藻糖磷酸酶 (T6PP) 催化 6-磷酸海藻糖 (T6P) 脱磷酸化为二糖海藻糖。该酶不存在于哺乳动物体内,但对多种低等生物的生存至关重要,因为海藻糖是一种关键的代谢物,而 T6P 的积累是有毒的。因此,T6PP 是治疗由细菌、真菌和寄生线虫引起的人类病理学的靶标。在这里,我们报告了载脂蛋白形式的鼠伤寒沙门氏菌T6PP ( St T6PP)以及与辅助因子 Mg 2+和底物类似物海藻糖 6-硫酸盐 (T6S)、产品海藻糖或竞争性复合物的X 射线晶体结构抑制剂 4-正辛基苯基α- d-吡喃葡萄糖苷 6-硫酸盐 (OGS)。OGS 用硫酸基取代底物磷酰基,用辛基苯基部分取代硫酸基远端的葡糖基环。这些具有 T6PP 的底物类似物和产物复合物的结构表明,特异性是通过氢键通过 Glu123、Lys125 和 Glu167 与磷酰基部分近端的葡糖基结合而赋予的,这在来自多个物种的 T6PP 中是保守的。第一代抑制剂 OGS 的结构表明,它保留了硫酸基团和近端糖基环观察到的底物结合相互作用。OGS 辛基苯基部分以独特的方式结合,表明该亚位点可以耐受各种化学型。一起,











































 京公网安备 11010802027423号
京公网安备 11010802027423号