当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Copper Salts on Amide Hydrothermal Formation and Reactivity
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-08-04 , DOI: 10.1021/acsearthspacechem.0c00150 Xuan Fu 1 , Yiju Liao 1 , Alexandria Aspin 1 , Ziming Yang 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-08-04 , DOI: 10.1021/acsearthspacechem.0c00150 Xuan Fu 1 , Yiju Liao 1 , Alexandria Aspin 1 , Ziming Yang 1
Affiliation
|
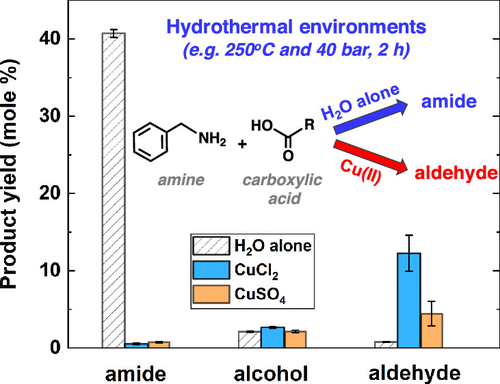
Unlike ambient conditions, water at elevated temperature and pressure can increasingly favor dissolution of hydrophobic organic molecules, act as an acid/base catalyst, and trigger unique hydrothermal pathways. In a recent study, we found that amides could be readily synthesized from simple amines and carboxylic acids in hydrothermal solutions, which implied a potential peptide and biomolecule synthetic pathway in natural hydrothermal environments. Formation of amide or peptide bonds is critical in producing building blocks of peptides and proteins, which are essential to living organisms, such as extremophiles, in hydrothermal systems. However, amide formation and degradation pathways under hydrothermal conditions are still not well understood, particularly how these pathways would be influenced by surrounding minerals or dissolved metals is unknown. Here, we describe the effects of copper(II) salts, such as copper chloride and copper sulfate, on the hydrothermal formation and reactivity of amides at 250 °C and 40 bar (Psat). The copper salts were chosen to be studied because they are commonly distributed in seawater and hydrothermal fluids. We found that the copper salts greatly inhibited the amide formation, with a decreased amide yield by up to 90% within 2 h. We also observed that aldehydes were quickly formed through the oxidation of amines by copper and became the dominant products. Compared to copper chloride/sulfate, copper acetate also facilitated the amine oxidation but did not suppress the amide formation, suggesting different roles of copper salts in amide hydrothermal synthesis. In addition, we found that hydrothermal reactivity of amides was strongly dependent on the solution pH, with lower reactivity at neutral pH than at very acidic or alkaline conditions. Our findings thus suggest that both copper and pH are important factors for amide hydrothermal synthesis and reactivity, which provide new insights into the prediction of amide or peptide bond synthesis in natural hydrothermal environments.
中文翻译:

铜盐对酰胺水热形成和反应性的影响
与环境条件不同,在升高的温度和压力下,水可以逐渐促进疏水性有机分子的溶解,充当酸/碱催化剂并触发独特的水热路径。在最近的研究中,我们发现在水热溶液中酰胺很容易由简单的胺和羧酸合成,这暗示了在自然水热环境中潜在的肽和生物分子合成途径。酰胺或肽键的形成对于产生肽和蛋白质的结构单元至关重要,而肽和蛋白质对热液系统中的活生物体(如极端微生物)至关重要。然而,在水热条件下酰胺的形成和降解途径仍未得到很好的理解,尤其是未知的是这些途径如何受到周围矿物或溶解金属的影响。在这里,我们描述了铜(II)盐(例如氯化铜和硫酸铜)在250°C和40 bar(P坐)。选择铜盐进行研究是因为它们通常分布在海水和热液中。我们发现铜盐极大地抑制了酰胺的形成,在2小时内酰胺的收率降低了90%。我们还观察到,醛通过铜被胺氧化而迅速形成,并成为主要产物。与氯化铜/硫酸铜相比,乙酸铜还促进胺的氧化,但不抑制酰胺的形成,表明铜盐在酰胺水热合成中的作用不同。此外,我们发现酰胺的水热反应性在很大程度上取决于溶液的pH值,在中性pH值下的反应性比在酸性或碱性条件下低。
更新日期:2020-09-18
中文翻译:

铜盐对酰胺水热形成和反应性的影响
与环境条件不同,在升高的温度和压力下,水可以逐渐促进疏水性有机分子的溶解,充当酸/碱催化剂并触发独特的水热路径。在最近的研究中,我们发现在水热溶液中酰胺很容易由简单的胺和羧酸合成,这暗示了在自然水热环境中潜在的肽和生物分子合成途径。酰胺或肽键的形成对于产生肽和蛋白质的结构单元至关重要,而肽和蛋白质对热液系统中的活生物体(如极端微生物)至关重要。然而,在水热条件下酰胺的形成和降解途径仍未得到很好的理解,尤其是未知的是这些途径如何受到周围矿物或溶解金属的影响。在这里,我们描述了铜(II)盐(例如氯化铜和硫酸铜)在250°C和40 bar(P坐)。选择铜盐进行研究是因为它们通常分布在海水和热液中。我们发现铜盐极大地抑制了酰胺的形成,在2小时内酰胺的收率降低了90%。我们还观察到,醛通过铜被胺氧化而迅速形成,并成为主要产物。与氯化铜/硫酸铜相比,乙酸铜还促进胺的氧化,但不抑制酰胺的形成,表明铜盐在酰胺水热合成中的作用不同。此外,我们发现酰胺的水热反应性在很大程度上取决于溶液的pH值,在中性pH值下的反应性比在酸性或碱性条件下低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号