当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Benzimidazole‐based fluorophores for the detection of amyloid fibrils with higher sensitivity than Thioflavin‐T
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-08-04 , DOI: 10.1111/jnc.15138 Narayanaperumal Pravin 1 , Rakesh Kumar 2, 3 , Shalini Tripathi 1 , Pardeep Kumar 1 , Ganesh M Mohite 2 , Ambuja Navalkar 2 , Rajlaxmi Panigrahi 2 , Namrata Singh 2 , Laxmikant G Gadhe 2 , Shaffi Manchanda 3 , Makoto Shimozawa 3 , Per Nilsson 3 , Jan Johansson 3 , Ashutosh Kumar 2 , Samir K Maji 2 , Maheswaran Shanmugam 1
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-08-04 , DOI: 10.1111/jnc.15138 Narayanaperumal Pravin 1 , Rakesh Kumar 2, 3 , Shalini Tripathi 1 , Pardeep Kumar 1 , Ganesh M Mohite 2 , Ambuja Navalkar 2 , Rajlaxmi Panigrahi 2 , Namrata Singh 2 , Laxmikant G Gadhe 2 , Shaffi Manchanda 3 , Makoto Shimozawa 3 , Per Nilsson 3 , Jan Johansson 3 , Ashutosh Kumar 2 , Samir K Maji 2 , Maheswaran Shanmugam 1
Affiliation
|
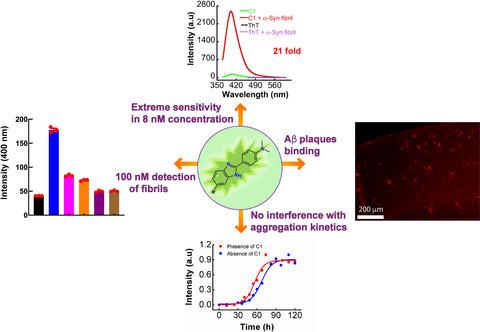
Protein aggregation into amyloid fibrils is a key feature of a multitude of neurodegenerative diseases such as Alzheimer's, Parkinson's, and Prion disease. To detect amyloid fibrils, fluorophores with high sensitivity and better efficiency coupled with the low toxicity are in high demand even to date. In this pursuit, we have unveiled two benzimidazole‐based fluorescence sensors ([C15H15N3] (C1) and [C16H16N3O2] (C2), which possess exceptional affinity toward different amyloid fibrils in its submicromolar concentration (8 × 10–9 M), whereas under a similar concentration, the gold standard Thioflavin‐T (ThT) fails to bind with amyloid fibrils. These fluorescent markers bind to α‐Syn amyloid fibrils as well as amyloid fibrils forming other proteins/peptides including Aβ42 amyloid fibrils. The 1H‐15N heteronuclear quantum correlation spectroscopy nuclear magnetic resonance data collected on wild‐type α‐Syn monomer with and without the fluorophores (C1 and C2) reveal that there is weak or no interactions between C1 or C2 with residues in α‐Syn monomer, which indirectly reflects the specific binding ability of C1 and C2 to the α‐Syn amyloid fibrils. Detailed studies further suggest that C1 and C2 can detect/bind with the α‐Syn amyloid fibril as low as 100 × 10–9 M. Extremely low or no cytotoxicity is observed for C1 and C2 and they do not interfere with α‐Syn fibrillation kinetics, unlike ThT. Both C1/C2 not only shows selective binding with amyloid fibrils forming various proteins/peptides but also displays excellent affinity and selectivity toward α‐Syn amyloid aggregates in SH‐SY5Y cells and Aβ42 amyloid plaques in animal brain tissues. Overall, our data show that the developed dyes could be used for the detection of amyloid fibrils including α‐Syn and Aβ42 amyloids with higher sensitivity as compared to currently used ThT.
中文翻译:

基于苯并咪唑的荧光团用于检测淀粉样蛋白原纤维的灵敏度比硫黄素T高
蛋白质聚集到淀粉样蛋白原纤维中是多种神经退行性疾病(例如阿尔茨海默氏病,帕金森氏病和Pri病毒病)的关键特征。为了检测淀粉样蛋白原纤维,迄今为止,仍要求高灵敏度和更好的效率以及低毒性的荧光团。为了实现这一目标,我们推出了两种基于苯并咪唑的荧光传感器([C 15 H 15 N 3 ](C1)和[C 16 H 16 N 3 O 2 ](C2),它们在其不同淀粉样蛋白原纤维上具有出色的亲和力亚微摩尔浓度(8×10 –9 M),而在相似浓度下,金标准硫黄素-T(ThT)无法与淀粉样蛋白原纤维结合。这些荧光标记物与α-Syn淀粉样蛋白原纤维以及形成其他蛋白质/肽(包括Aβ42淀粉样蛋白原纤维)的淀粉样蛋白原纤维结合。在具有和不具有荧光团(C1和C2)的野生型α-Syn单体上收集的1 H- 15 N异核量子相关光谱核磁共振数据表明,C1或C2与α-中的残基之间没有弱相互作用或没有相互作用顺式单体,间接反映C1和C2的特异性结合能力α-Syn淀粉样蛋白原纤维。详细的研究进一步表明,C1和C2可以检测/结合至低至100×10 –9 M的α-Syn淀粉样原纤维。C1和C2的细胞毒性极低或未观察到细胞毒性,并且它们不干扰α-Syn的原纤维化动力学,与ThT不同。两个C1 / C2不仅显示出与形成各种蛋白质/肽的淀粉样蛋白原纤维的选择性结合,而且还对SH-SY5Y细胞中的α-Syn淀粉样蛋白聚集体和动物脑组织中的Aβ42淀粉样蛋白斑显示出优异的亲和力和选择性。总体而言,我们的数据表明,与目前使用的ThT相比,开发的染料可用于检测包括α-Syn和Aβ42淀粉样蛋白在内的淀粉样蛋白原纤维。
更新日期:2020-08-04
中文翻译:

基于苯并咪唑的荧光团用于检测淀粉样蛋白原纤维的灵敏度比硫黄素T高
蛋白质聚集到淀粉样蛋白原纤维中是多种神经退行性疾病(例如阿尔茨海默氏病,帕金森氏病和Pri病毒病)的关键特征。为了检测淀粉样蛋白原纤维,迄今为止,仍要求高灵敏度和更好的效率以及低毒性的荧光团。为了实现这一目标,我们推出了两种基于苯并咪唑的荧光传感器([C 15 H 15 N 3 ](C1)和[C 16 H 16 N 3 O 2 ](C2),它们在其不同淀粉样蛋白原纤维上具有出色的亲和力亚微摩尔浓度(8×10 –9 M),而在相似浓度下,金标准硫黄素-T(ThT)无法与淀粉样蛋白原纤维结合。这些荧光标记物与α-Syn淀粉样蛋白原纤维以及形成其他蛋白质/肽(包括Aβ42淀粉样蛋白原纤维)的淀粉样蛋白原纤维结合。在具有和不具有荧光团(C1和C2)的野生型α-Syn单体上收集的1 H- 15 N异核量子相关光谱核磁共振数据表明,C1或C2与α-中的残基之间没有弱相互作用或没有相互作用顺式单体,间接反映C1和C2的特异性结合能力α-Syn淀粉样蛋白原纤维。详细的研究进一步表明,C1和C2可以检测/结合至低至100×10 –9 M的α-Syn淀粉样原纤维。C1和C2的细胞毒性极低或未观察到细胞毒性,并且它们不干扰α-Syn的原纤维化动力学,与ThT不同。两个C1 / C2不仅显示出与形成各种蛋白质/肽的淀粉样蛋白原纤维的选择性结合,而且还对SH-SY5Y细胞中的α-Syn淀粉样蛋白聚集体和动物脑组织中的Aβ42淀粉样蛋白斑显示出优异的亲和力和选择性。总体而言,我们的数据表明,与目前使用的ThT相比,开发的染料可用于检测包括α-Syn和Aβ42淀粉样蛋白在内的淀粉样蛋白原纤维。











































 京公网安备 11010802027423号
京公网安备 11010802027423号