当前位置:
X-MOL 学术
›
J. Neurosci. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impaired intracellular trafficking of sodium-dependent vitamin C transporter 2 contributes to the redox imbalance in Huntington's disease.
Journal of Neuroscience Research ( IF 2.9 ) Pub Date : 2020-08-04 , DOI: 10.1002/jnr.24693 Adriana Covarrubias-Pinto 1 , Alejandra V Parra 1 , Gonzalo Mayorga-Weber 1 , Eduardo Papic 1 , Isidora Vicencio 1 , Pamela Ehrenfeld 2, 3 , Francisco J Rivera 2, 3, 4, 5 , Maite A Castro 1, 2, 6
Journal of Neuroscience Research ( IF 2.9 ) Pub Date : 2020-08-04 , DOI: 10.1002/jnr.24693 Adriana Covarrubias-Pinto 1 , Alejandra V Parra 1 , Gonzalo Mayorga-Weber 1 , Eduardo Papic 1 , Isidora Vicencio 1 , Pamela Ehrenfeld 2, 3 , Francisco J Rivera 2, 3, 4, 5 , Maite A Castro 1, 2, 6
Affiliation
|
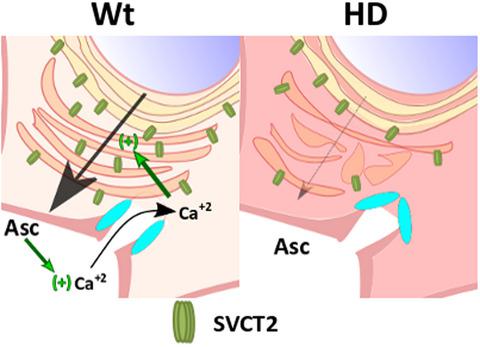
Huntington's disease (HD) is a neurodegenerative disorder caused by a glutamine expansion at the first exon of the huntingtin gene. Huntingtin protein (Htt) is ubiquitously expressed and it is localized in several organelles, including endosomes. HD is associated with a failure in energy metabolism and oxidative damage. Ascorbic acid is a powerful antioxidant highly concentrated in the brain where it acts as a messenger, modulating neuronal metabolism. It is transported into neurons via the sodium‐dependent vitamin C transporter 2 (SVCT2). During synaptic activity, ascorbic acid is released from glial reservoirs to the extracellular space, inducing an increase in SVCT2 localization at the plasma membrane. Here, we studied SVCT2 trafficking and localization in HD. SVCT2 is decreased at synaptic terminals in YAC128 male mice. Using cellular models for HD (STHdhQ7 and STHdhQ111 cells), we determined that SVCT2 trafficking through secretory and endosomal pathways is altered in resting conditions. We observed Golgi fragmentation and SVCT2/Htt‐associated protein‐1 mis‐colocalization. Additionally, we observed altered ascorbic acid‐induced calcium signaling that explains the reduced SVCT2 translocation to the plasma membrane in the presence of extracellular ascorbic acid (active conditions) described in our previous results. Therefore, SVCT2 trafficking to the plasma membrane is altered in resting and active conditions in HD, explaining the redox imbalance observed during early stages of the disease.
中文翻译:

钠依赖性维生素 C 转运蛋白 2 的细胞内运输受损导致亨廷顿病的氧化还原失衡。
亨廷顿病 (HD) 是一种神经退行性疾病,由亨廷顿基因第一个外显子的谷氨酰胺扩增引起。亨廷顿蛋白 (Htt) 无处不在,它位于多种细胞器中,包括内体。HD 与能量代谢和氧化损伤的失败有关。抗坏血酸是一种强大的抗氧化剂,高度集中在大脑中,作为信使,调节神经元代谢。它通过钠依赖性维生素 C 转运蛋白 2 (SVCT2) 转运到神经元中。在突触活动期间,抗坏血酸从神经胶质库释放到细胞外空间,导致 SVCT2 在质膜上的定位增加。在这里,我们研究了 HD 中的 SVCT2 贩运和定位。YAC128 雄性小鼠的突触末端 SVCT2 减少。使用 HD 细胞模型(STHdhQ7 和 STHdhQ111 细胞),我们确定通过分泌和内体途径运输的 SVCT2 在静息条件下发生改变。我们观察到高尔基体片段化和 SVCT2/Htt 相关蛋白 1 错误共定位。此外,我们观察到抗坏血酸诱导的钙信号发生改变,这解释了在我们之前的结果中描述的细胞外抗坏血酸(活性条件)存在的情况下,SVCT2 向质膜的易位减少。因此,运输至质膜的 SVCT2 在 HD 的静息和活动条件下发生改变,解释了在疾病早期阶段观察到的氧化还原失衡。我们观察到高尔基体片段化和 SVCT2/Htt 相关蛋白 1 错误共定位。此外,我们观察到抗坏血酸诱导的钙信号发生改变,这解释了在我们之前的结果中描述的细胞外抗坏血酸(活性条件)存在的情况下,SVCT2 向质膜的易位减少。因此,运输到质膜的 SVCT2 在 HD 的静息和活动条件下发生改变,解释了在疾病早期阶段观察到的氧化还原失衡。我们观察到高尔基体片段化和 SVCT2/Htt 相关蛋白 1 错误共定位。此外,我们观察到抗坏血酸诱导的钙信号发生改变,这解释了在我们之前的结果中描述的细胞外抗坏血酸(活性条件)存在的情况下,SVCT2 向质膜的易位减少。因此,运输到质膜的 SVCT2 在 HD 的静息和活动条件下发生改变,解释了在疾病早期阶段观察到的氧化还原失衡。
更新日期:2020-08-04
中文翻译:

钠依赖性维生素 C 转运蛋白 2 的细胞内运输受损导致亨廷顿病的氧化还原失衡。
亨廷顿病 (HD) 是一种神经退行性疾病,由亨廷顿基因第一个外显子的谷氨酰胺扩增引起。亨廷顿蛋白 (Htt) 无处不在,它位于多种细胞器中,包括内体。HD 与能量代谢和氧化损伤的失败有关。抗坏血酸是一种强大的抗氧化剂,高度集中在大脑中,作为信使,调节神经元代谢。它通过钠依赖性维生素 C 转运蛋白 2 (SVCT2) 转运到神经元中。在突触活动期间,抗坏血酸从神经胶质库释放到细胞外空间,导致 SVCT2 在质膜上的定位增加。在这里,我们研究了 HD 中的 SVCT2 贩运和定位。YAC128 雄性小鼠的突触末端 SVCT2 减少。使用 HD 细胞模型(STHdhQ7 和 STHdhQ111 细胞),我们确定通过分泌和内体途径运输的 SVCT2 在静息条件下发生改变。我们观察到高尔基体片段化和 SVCT2/Htt 相关蛋白 1 错误共定位。此外,我们观察到抗坏血酸诱导的钙信号发生改变,这解释了在我们之前的结果中描述的细胞外抗坏血酸(活性条件)存在的情况下,SVCT2 向质膜的易位减少。因此,运输至质膜的 SVCT2 在 HD 的静息和活动条件下发生改变,解释了在疾病早期阶段观察到的氧化还原失衡。我们观察到高尔基体片段化和 SVCT2/Htt 相关蛋白 1 错误共定位。此外,我们观察到抗坏血酸诱导的钙信号发生改变,这解释了在我们之前的结果中描述的细胞外抗坏血酸(活性条件)存在的情况下,SVCT2 向质膜的易位减少。因此,运输到质膜的 SVCT2 在 HD 的静息和活动条件下发生改变,解释了在疾病早期阶段观察到的氧化还原失衡。我们观察到高尔基体片段化和 SVCT2/Htt 相关蛋白 1 错误共定位。此外,我们观察到抗坏血酸诱导的钙信号发生改变,这解释了在我们之前的结果中描述的细胞外抗坏血酸(活性条件)存在的情况下,SVCT2 向质膜的易位减少。因此,运输到质膜的 SVCT2 在 HD 的静息和活动条件下发生改变,解释了在疾病早期阶段观察到的氧化还原失衡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号